Magnetic resonance spectroscopy metabolomics of cerebrospinal fluid in patients with multiple sclerosis, clinically isolated syndrome, other inflammatory brain diseases and controls
Metabolomika cerebrospinálneho likvoru pomocou magnetickej rezonančnej spektroskopie u pacientov so sclerosis multiplex, s klinicky izolovaným syndrómom, inými zápalovými ochoreniami mozgu a u zdravých kontrol
Cieľ: Včasné rozpoznanie sclerosis multiplex (SM) pomáha začať liečbu pacientov skôr, a tak oddialiť progresiu ochorenia. Urobili sme analýzu metabolitov cerebro-spinálneho likvoru (cerebrospinal fluid; CSF), s cieľom zistiť prediktory včas, a tak oddialiť SM.
Metódy: Do štúdie bolo zaradených 56 jedincov s podozrením na SM, pred začatím akejkoľvek liečby. Z nich bolo 28 diagnostikovaných ako definitívna SM, u 17 pacientov sme zistili klinicky izolovaný syndróm (clinically isolated syndrome; CIS) podľa McDonaldových kritérií z roku 2010, v 11 prípadoch sa jednalo o iné demyelinizačné ochorenie CNS (DEM). Kontrolnú skupinu (CON) tvorili 29 jedinci, ktorí nemali dokázané žiadne ochorenie CNS. Na meranie metabolitov CSF bola použitá protonová nukleárna magnetická rezonančná spektroskopia.
Výsledky: Glutamín, ktorý koreloval s Expanded Disability Status Scale (EDSS), bol jediným metabolitom, ktorý dokázal odlíšiť CIS, SM, DEM a CON. Valín, leucín, isoleucín, znížené u CIS a SM v porovnaní s CON, sa neodlišovali od DEM. Hladiny citrátu v CSF špecifikovali SM a CIS oproti DEM, ale nepomohli v rozlíšení CIS a SM. Citrát ukazoval signifikantné korelácie s vekom, dľžkou trvania ochorenia a EDSS u SM pacientov. Acetát, aceton, pyruvát, formát, histidin v CSF neboli signifikantnými prediktormi SM alebo CIS, hoci korelovali s niektorými vybranými premennými.
Záver: Táto práca ukazuje prediktívnu úlohu glutamínu v CSF v stanovení diagnózy SM od jej včasných štádií, vypichujúc tak dôležitú úlohu glutamát/glutamínového cyklu v patogenéze SM. Ďalší potenciálny prediktor SM bol citrát. Ďalšie metabolity neboli identifikované ako senzitívne CSF markery SM.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Klíčová slova:
cerebro-spinálny likvor – metabolomika – jednoprotonová nukleárna magnetická rezonančná spektroskopia (1H-NMRS) – sclerosis multiplex – klinicky izolovaný syndrom – zápalové demyelizačné ochorenia mozgu
Authors:
E. Baranovičová 1; D. Čierny 2; P. Hnilicova 1; J. Lehotský 1,3; R. Murín 3; Š. Sivák 4; E. Kurča 4; L. Plicová 2; E. Kantorová 4
Authors‘ workplace:
BioMedical Center BioMed, Division, of Neurosciences, Jessenius Faculty, of Medicine, Comenius University in, Bratislava, Martin, Slovakia
1; Department of Clinical Biochemistry, Jessenius Faculty of Medicine, Comenius University in Bratislava and, University Hospital Martin, Slovakia
2; Department of Medical Biochemistry, Jessenius Faculty of Medicine, Comenius University in Bratislava, Martin, Slovakia
3; Clinic of Neurology, Jessenius Faculty, of Medicine, Comenius University, in Bratislava and University Hospital, Martin, Slovakia
4
Published in:
Cesk Slov Neurol N 2020; 83/116(3): 315-322
Category:
Original Paper
doi:
https://doi.org/10.14735/amcsnn2020315
Overview
Aim: Early recognition of multiple sclerosis (MS) allows patients to begin treatment earlier and delay disease progression. We performed an analysis of cerebrospinal fluid (CSF) metabolites to find early predictors of MS.
Methods: We included 56 participants with suspected MS before any treatment. Out of those, 28 patients were diagnosed with definite MS, 17 with clinically isolated syndrome (CIS) according to McDonald 2010 criteria, and 11 with other demyelinating diseases (DEM) of the CNS. The control group (CON) included 29 participants without any confirmed CNS disease. Proton nuclear magnetic resonance spectroscopy was used to measure CSF metabolites.
Results: Glutamine, correlating with Expanded Disability Status Scale (EDSS), was the only metabolite capable to distinguish between CIS and MS, DEM, and CON. Valine, leucine, isoleucine, decreased in CIS and MS when compared with CON, did not differ from DEM. Citrate CSF levels specified MS and CIS against DEM but did not help to distinguish between CIS and MS. Citrate showed significant correlations with age, disease duration, and EDSS in MS patients. Acetate, acetone, pyruvate, formate and histidine CSF levels were not significant predictors of MS or CIS, although they correlated with selective variables.
Conclusion: This work shows the predictive role of CSF glutamine in diagnosing MS since its early stages, pinpointing an important role of the glutamate/glutamine cycle in MS pathogenesis. Another potential predictor of MS was citrate. Other metabolites were not identified as sensitive CSF markers of MS.
Keywords:
Multiple sclerosis – clinically isolated syndrome – Metabolomics – cerebrospinal fl uid – proton nuclear magnetic resonance spectroscopy (1H-NMRS) – infl ammatory demyelinating brain diseases
Introduction
Multiple sclerosis (MS) is a heterogeneous disease with an unpredictable disease course. It is known that accumulating disability reflects the progression of neuronal damage, which can occur during clinically silent inflammatory episodes. Neurodegeneration was traditionally considered as a secondary phenomenon to inflammation and demyelination. However, recent data indicate that neurodegeneration develops along with inflammation and demyelination [1]. While both inflammation and demyelination are well described and understood cellular processes, neurodegeneration is still mattered of debate.
In MS, our current treatments reduce inflammation and, therefore relapses and short-term disability, and we hope this will slow-down the rate of neurodegeneration [2]. Studies that have analyzed early treatment in patients highly likely to have MS (clinically isolated events with the evidence of lesions on brain MRI) provided significant benefits in delaying further changes in MRI and attacks [2,3]. Early recognition of the inflammatory process enables patients to begin treatment with an immunomodulatory agent but may also increase the rate of false--positive MS diagnoses [3]. However, in MS, there is no such specific test available which is why one needs to rely on “circumstantialevidence”. The diagnosis is based on typical, yet not limited to, clinical findings, MRI, and cerebrospinal fluid (CSF) examination as well as other investigations [4].
In our previous work, we found superiority of neurodegenerative metabolic pathways in brain tissue of MS patients using the in vivo proton nuclear magnetic resonance spectroscopy (1H-NMRS) method [5]. Now we decided to test CSF metabolites as we believe that additional molecules of CSF could help to improve the differential diagnosis of MS and its subtypes and predict the course of the disease, thus selecting optimal therapy for each patient.
Both inflammatory and neurodegenerative markers of CSF have yet been described [6]. However, so far, only several groups of researchers studied concentrations of metabolomics in CSF using 1H-NMRS [7–13]. Nevertheless, the results are partly inconsistent.
According to our knowledge, our work is the first one presenting comparative analysis of CSF metabolites to find early predictors of MS.
Materials and methods
Patients and controls
Informed written consent was obtained from all study participants after approving the study protocol by the local ethics committee. Only participants with suspected MS before any treatment were included. The patients underwent clinical and paraclinical examinations to prove or exclude MS. Clinical disability was evaluated by 2 neurologists specialized in MS using Expanded Disability Status Scale (EDSS). The neurologists have been Neurostatus certified in EDSS evaluation. MRI examination of the brain and cervical spinal cord performed according to the approved protocol (MAGNIMS) provided evidence of pathological lesions and their activity. Evoked potentials (visual, acoustic, somatosensory tests) informed about potential demyelinating and axonal changes of the tracts.
CSF and blood samples were used for biochemical and immunological tests. In CSF, we tested oligoclonal bands (OCB), flow cytometry, immunoglobulins, and selectively antibodies against autoimmune encephalitis. MS and CIS diagnosis were based on the McDonald criteria 2010 [3]. Based on the results, 28 patients were diagnosed with definite MS, 17 with CIS (patients with MS after the first clinical relapse), and 11 with other inflammatory brain diseases of the CNS (demyelinating diseases of the CNS; DEM). These patients presented with either isolated monophasic optic neuritis or with idiopathic myelitis, vasculitis or monophasic forms of the CNS demyelination. MRI lesions were different from MS and did not comply with the Barkhof criteria for MS [14]. In this group, OCB were negative (type 1). The patients also did not fulfill MRI criteria for neuromyelitis optica, and IgG antibodies against aquaporin-4 were negative. Three out of the patients were tested to anti-myelin oligodendrocyte glycoprotein (anti-MOG) with negative results.
Due to ethical concerns surrounding the collection of CSF from healthy individuals, healthy controls were not recruited for this study. The control group (CON) included 29 participants without any confirmed CNS disease. These were patients with episodic severe headache, lower back pain or peripheral neuropathy, and normal MRI scans of the brain and spinal cord. In these patients, lumbar punction and evaluation of CSF samples were indicated to exclude intracranial bleeding, neuroinfections, or a disorder of lower motoneuron, e. g., peripheral neuropathy. Biochemical tests were indicated in all samples, and immunological tests were applied in selected CFS samples accordingly.
Sample preparation
The stock solution consisted of phosphate buffer 250 mL and 0.28 mMTMSP-d4 (trimethylsilylpropionic acid -d4) as a chemical shift reference in deuterated water. CSF was immediately centrifuged at 2,000 rpm at 4º C for 20 min and then frozen to –80 ºC until examined. After thawing, 500 μL of CSF was softly mixed with a 100 μL stock solution and transferred into a 5 - mm 1H-NMR tube. Samples were randomized for acquisition.
1H-NMRS data acquisition
1H-NMRS data were acquired on 600 MHz 1H-NMRS spectrometer Avance III (Bruker, Billerica, MA, USA) equipped with cryoprobe at T = 310 K. Initial settings (basal shimming, receiver gain, water suppression frequency) was done on an independent sample and adopted for measurements. After the preparation, samples were stored in a Sample Jet, cooled at 5 ºC. Before the measurement, each sample was preheated on the 310 K for 5 min. An exponential noise filter was used to introduce 0.3-Hz line broadening before Fourier transform. The TMSP-d4 signal was assigned a chemical shift of 0.000 ppm.
We modified the standard profiling protocols from Bruker as follows: NOESY with presaturation: FID size 64k, dummy scans 4, number of scans 128, spectral width 20.4750 ppm; COSY with presaturation: FID size 4k, dummy scans 8, number of scans 1, spectral width 16.0125 ppm; homonuclear J-resolved: FID size 8k, dummy scans 16, number of scans 4; CPMG with pre-saturation: FID size 64k, dummy scans 4, number of scans 128, spectral width 20.0156 ppm. All experiments were conducted with a relaxation delay of 5 s.
Data analysis
All spectra were binned to bins of the size of 0.001 ppm, starting from 0.500 ppm to 9.000 ppm, with an excluded water region of 4.6–4.9 ppm. Spectra were solved with the help of the human metabolomics database, chenomics software and by researching in metabolomics literature. For all compounds, the multiplicity of peaks was confirmed in j-resolved spectra and homonuclear cross peaks were confirmed in COSY spectra. After the metabolites were identified (Fig. 1), we chose spectra subregions with only a single metabolite assigned, or minimally affected by other cometabolites. In 0.001 ppm binned spectra, we summed integrals of selected signals. Metabolites showing weak intensive peaks or strong peak overlap were excluded from the evaluation. Statistical tests were performed in Matlab R2015a (MathWorks, Naticks, MA, USA).
Obr. 1. 1H-NMR spektrum v cerebrospinálnom likvore, nábor v pulzovej sekvencii 1H-NMR.
1 – izoleucín; 2 – leucín; 3 – valín; 4 – laktát; 5 – alanín; 6 – acetát; 7 – glutamín; 8 – acetón;
9 – pyruvát; 10 – citrát; 11 – kreatin; 12 – kreatinín; 13 – myo-inozitol; 14 – glukóza; 15 – tyrozín; 16 – histidín; 17 – fenylalanín; 18 – formát
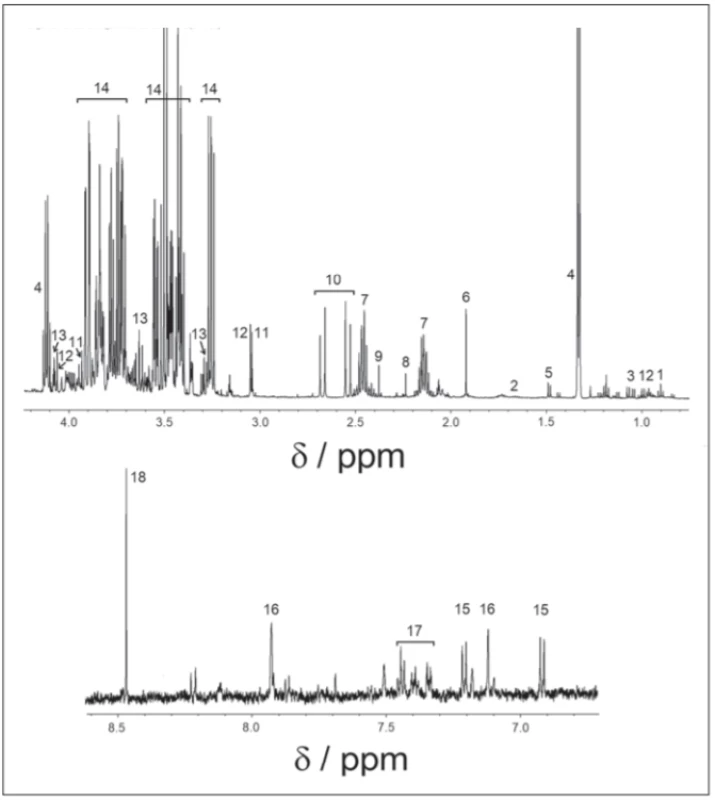
Results
Finally, 18 metabolites: valine, isoleucine, leucine (branched-chain amino acids [BCAA]), lactate, alanine, formate, tyrosine, phenylalanine, glutamine, histidine, citrate, acetate, acetone, pyruvate, myoinositol, glucose, creatine and creatinine were identified in 1H-NMRS spectra.
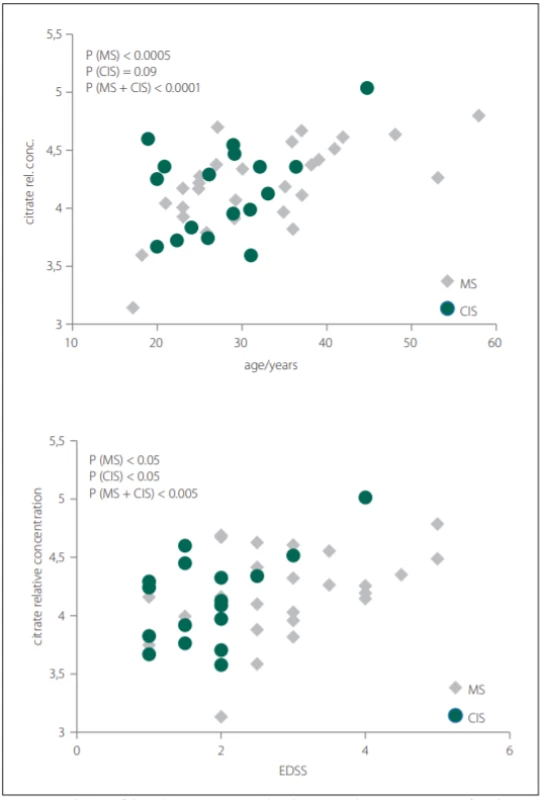
Glutamine was the only metabolite which levels differed between CIS, MS and CON. Glutamine also correlated with EDSS in MS and CIS. In CSF, BCCA (valine, leucine, isoleucine) were decreased in CIS/MS patients after the first clinical relapse and MS compared with CON and also correlated with age and disease duration (DD) of MS patients. Citrate CSF levels showed signifi-cant correlations with age, DD and EDSS in MS patients (Fig. 2). The acetate CSF levels were significantly increased in MS against all other groups. Other CSF metabolites significantly different between groups were formate, histidine, acetone, and pyruvate.
Obr. 2. Korelácia relatívnych hladín citrátu v cerebrospinálnom likvore s vekom a skóre
EDSS pacientov s MS a CIS.
CIS – klinicky izolovaný syndróm; CSF – cerebrospinálny likvor; EDSS – Expanded Disability
Status Scale; MS – sclerosis multiplex
Correlations of relative metabolites CSF levels between subgroups are summarized in Tab. 1. Results from statistic calculation related to patients parameters are summarized in Tab. 2.
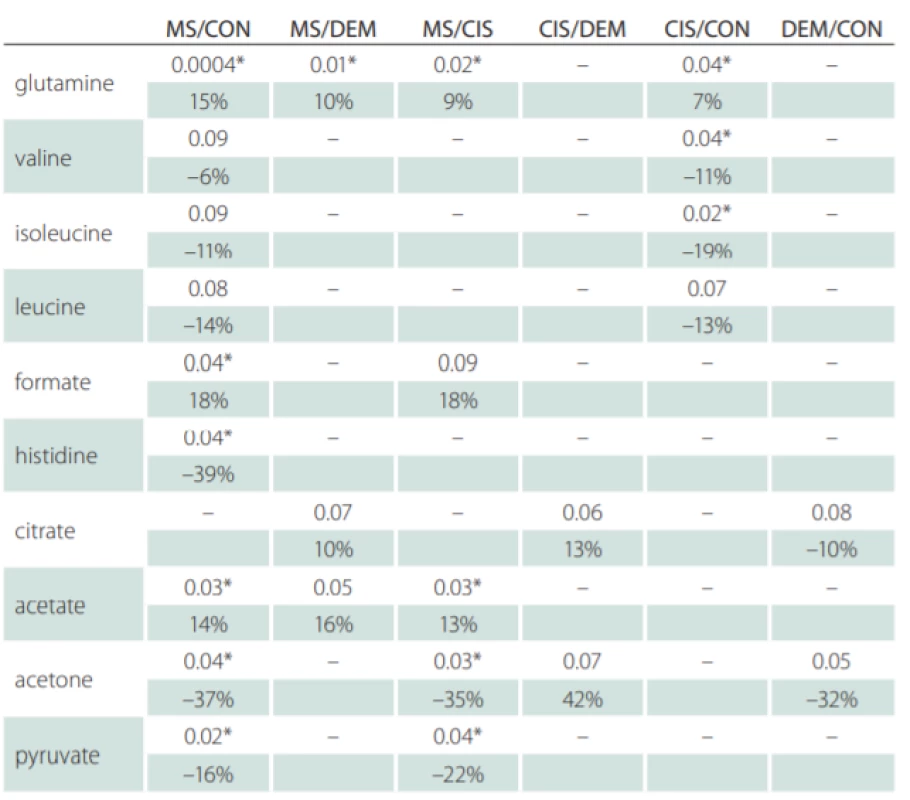
CON – controls; CIS/MS – patients after the first clinical relapse (clinically isolated syndrome vs.
multiple sclerosis); DEM – other infl ammatory brain diseases; MS – multiple sclerosis
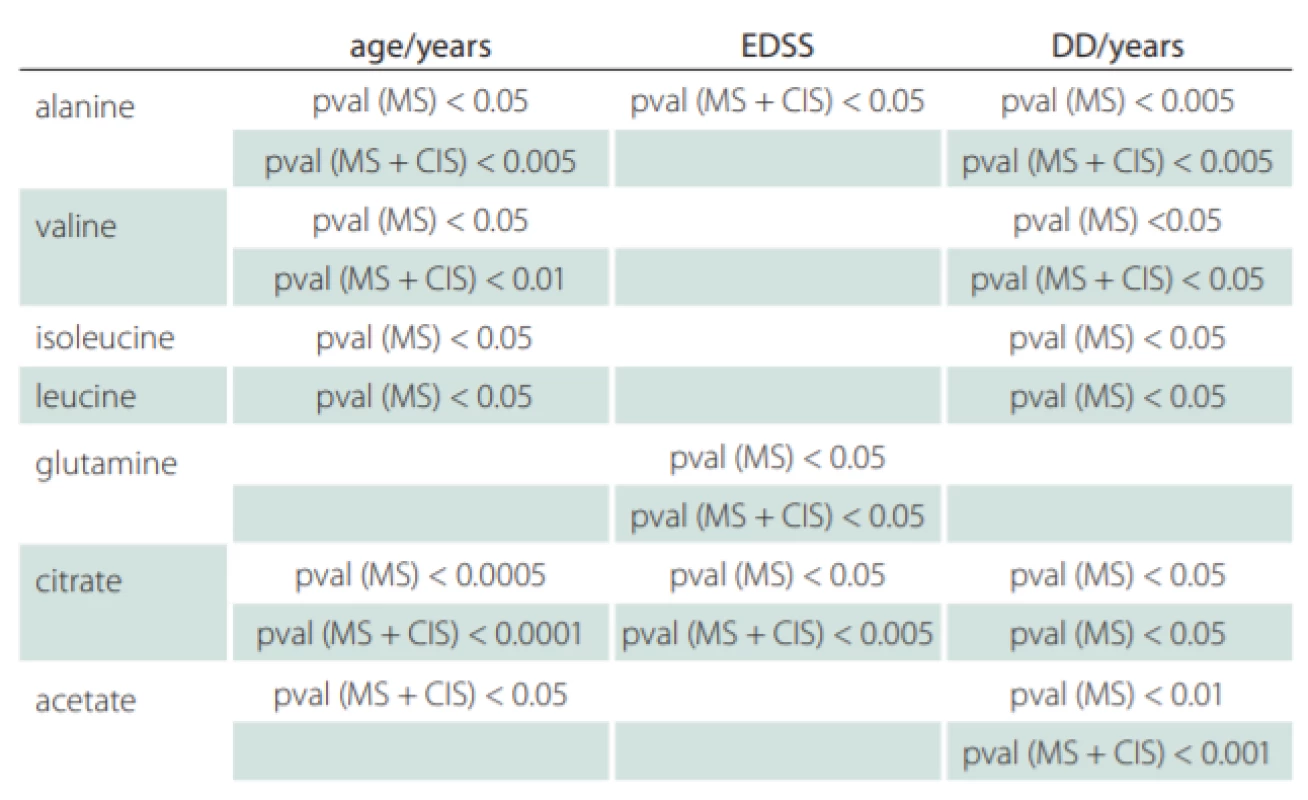
CIS – clinically isolated syndrome (patients after the first clinical relapse); DD – disease duration in years; EDSS – Expanded Disability Status Scale; MS – multiple sclerosis
Discussion
Glutamine
Glutamine is an ubiquitous amino acid in mammalian tissues and in the blood, where it is a precursor or product in multiple metabolic pathways in both the CNS and in the peripheral tissues. Glutamine participates in a glutamine-glutamate cycle that plays a role in neuroglia communication in the synapse. After the release of neurotransmitter glutamate into the synapse, this is rapidly up-taken by astrocytes and metabolized to glutamine, which is delivered into neurons, metabolized back to glutamate and repeatedly used for neurotransmission [15]. Glutamate, the principal CNS excitatory neurotransmitter, is the most abundant amino acid in the brain with the extracellular concentration much lower than the intracellular one [16]. There is growing evidence that glutamate plays a role in the pathology of MS. Increased glutamate CSF levels were observed in MS patients by Bakhartova et al [17] and Sarchielli et al [18] and correlated with disease severity and course [18].
Glutamine crosses the blood–brain barrier (BBB) via facilitating transport systems [19], using its carrier [20]. The concentration of glutamine in the CSF is very close to its concentrations in the arterial blood or extracellular fluid [21]. In our work, we observed increased CSF levels of glutamine, co-metabolite of glutamate, in patients with MS and in CIS/MS patients after the first clinical relapse when compared to CON and DEM. In our patients, the transmission of glutamine from the arterial blood to the CSF via hyperpermeable BBB cannot be rejected, but this mechanism does not explain a selective increase of glutamine only in MS and CIS/MS patients after the first clinical relapse over other forms of brain diseases, where disruption of the BBB is also found [22]. We supposed the active transformation of glutamate to glutamine inside the brain of MS and CIS/MS patients after the first clinical relapse.
MS is considered to be the mostly T-cell mediated disease, and T-cell activation is associated with abnormal glutamate/glutamine functioning. T-cell activation is dependent on extracellular glutamine and T-cell activation induces expression of glutamine transporters [23], which has also been proven in the peripheral blood [24]. The depletion of glutamine blocks proliferation and cytokine production and this cannot be rescued by supplying biosynthetic precursors of glutamine. T-cells are located at the active edge of MS lesions, and the presence of perivascular T-cell infiltrates throughout the CNS is a consistent feature in the early stages of MS [25]. Although var-ious processes involving glutamine are well described, it is unknown, if increased glutamine levels represent rather the cause or the consequence in developing CIS and further MS. Based on our results, we assume its participation in disease development, since the glutamine CSF level was increased in MS patients over CIS (MS patients after a first clinical relapse), in MS and CIS (MS patients after a first clinical relaps) over CON, and it correlated with the degree of disability.
Contrary to our results, Ashley et al [10] reported a decreased CSF glutamine level in MS against controls. However, the investigation in highly homogeneous CIS patient cohorts produced results with increased CSF glutamine levels [7]. Increased levels of glutamine in the CSF were also documented in different forms of neurodegeneration such as in Alzheimer disease [26] or amyotrophic lateral sclerosis [27]. Further examination in this field is required to elucidate links between glutamate and different types of neurodegenerative disorders.
Leucine, isoleucine, valine (BCAA)
The branched-chain amino acids leucine, isoleucine and valine cross the BBB swiftly. It seems probable that BCAA participate in a “glutamate-BCAA cycle”, which involves shuttling BCAA between astrocytes and neurons [28]. The metabolism of BCAA in glial and neural cells was studied in detail by Murin et al [29–31]. Besides function as “buffering” of an internal pool of glutamate, BCAA influence the immune properties of microglial cells and their responsiveness to pro-inflammatory signals in vitro [32].
Valine, leucine and isoleucine CSF levels were found decreased in MS, CIS (MS patients after the first clinical relapse) and DEM against the CON group (Tab. 1) and correlated positively with DD and age of MS patients (Tab. 2). Since these essential amino acids are transported into the brain by crossing BBB via controlled, facilitated transport and because they are used for accelerated glutamate synthesis, their reduced CSF levels may indicate their partial depletion due to this process.
Citrate
There is insufficient knowledge about specific CNS processes in which citrate may play a prominent role. In general, citrate is the key metabolite of Krebs cycle. Besides this, citrate plays an important role in the fatty-acid synthesis, the main component of brain lipids [33]. Citrate is released from astrocytes in large amounts, which is in keeping with high concentrations found in the CSF [34]. However, the functional importance of high concentrations of citrate in the CSF and the large release of citrate from astrocytes are not fully understood. Rather than citrate serving as a precursor for the synthesis of transmitter glutamate, citrate released from astrocytes may regulate the extracellular concentrations of Ca2+, Mg2+ and Zn2+ by chelation [35], thereby modulating neuronal excitability as an endogenous modulator of glutamate receptors, in particular, the N-methyl-D-aspartate (NMDA) receptor subtype. Citrate has a preference for Zn2+ over Ca2+ and Mg2+ [35] and is potentially able to abolish the inhibitory action of Zn2+ of the NMDA receptors in a reverse manner [36]. It is therefore attractive to suggest a hitherto unknown regulatory function of citrate in particular astrocytes [37].
In our study, we did not observe significant differences in citrate CSF levels between patient and control groups, contrary to other results, where decreased CSF citrate level was found in MS patients [7,38]. However, we observed the relationship of citrate CSF level to several clinical parameters of MS and CIS (Tab. 2). The CSF levels of citrate increased with EDSS score, DD and age in MS and with EDSS score and age in CIS (MS patients after the first clinical relapse) (Fig. 2).
Glucose, lactate, and pyruvate
Astrocytes have the highest concentration of the primary glucose transporter. Glucose, transported into astrocyte, is entering glycolysis with pyruvate as a product. Pyruvate has two fates: it can enter Krebs cycle in mitochondria or it is converted to lactate, which is then transported to neurons. Once lactate enters neurons, it is converted back to pyruvate. Neuronal pyruvate can be shuttled to mitochondria and funneled into Krebs cycle to gain energy or convert to citrate for lipid synthesis. Neurons show a relative preference for lactate over glucose when both substrates are present. However, we do not yet know the relative contributions of glycolysis and oxidative phosphorylation to the immediate energy demands of the neurons.
In our study, CSF levels of glucose and lactate were not changed in any of the groups. Based on our results, it seems that neither “lactate shuttle” nor glycolysis was affected in the evaluated groups. Other studies published conflicting results in the CSF in MS: decreased glucose [9], increased glucose [39], lactate without change [9], increased lactate [7] and decreased lactate [40]. We found decreased CSF pyruvate levels in MS patients against CIS (MS patients after the first clinical relapse) and CON. Current research revealed many mitochondrial abnormalities involved in the development and progression of MS [41] that are also supposed in our MS patients. Decreased pyruvate may also balance mitochondrial citrate overproduction in an advanced disease.
Histidine
Histidine is a precursor of histamine, a neurotransmitter for the brain and spinal cord. Elevated CSF histamine levels in MS patients were already reported [42,43]. Histamine can be found in large amounts in granules of mast cells, a type of white blood cells, and in granulocytes, derived from myeloid stem cells. Mast cells participate in innate and adaptive immunity, inflammation and autoimmunity [44]. We observed decreased histidine CSF levels in MS patients when compared to CON. Considering that histamine is produced from histidine by irreversible decarboxylation and histidine is an essential amino acid for the brain, entering the brain via BBB transporter systems, the increased histamine can be manifested by the exhaustion of histidine.
Acetate and formate
Previous data showed that levels of acetate and formate in MS patients were altered [9,39,45]. Our analysis revealed statistically significantly increased levels of both acetate and formate in CSF of MS patients against CON, CIS patients, and DEM (Tab. 1).
In general, the majority of acetate is generated by the enzymatic hydrolysis of acetyl-CoA. Acetyl-CoA is a co-substrate for the synthesis of citrate, and therefore sustaining the reactions of the citric acid cycle has a profound impact on the energy metabolism and biosynthetic reactions of fatty acids, cholesterol, ketone bodies and non-essential amino acids and their derivates including neurotransmitters glutamate, gamma-aminobutyric acid (GABA) and acetylcholine. Furthermore, acetyl-CoA is a common donor of acetyl residue for the synthesis of N-acetylaspartate or N-acetylaspartylglutamate; and also is essential for the sustaining of protein acetylation [46]. Reactivation of acetate into acetyl-CoA may take place in cytosolic, mitochondrial and nuclear compartments of glial cells and neurons. The cytosolic expression of acetyl-CoA synthase in oligodendrocytes [47] suggests their capability to utilize acetate for lipid synthesis and myelination [46].
Formate may be generated either by peroxisomal a-oxidation of fatty acids [48] or disturbed mitochondrial metabolism [49]. Since brain lipids consist of a considerable proportion of fatty acids [33], the demyelination accompanying lipolysis can elevate the level of free fatty acids, and then formate. In the brain, acetate and formate are further metabolized in glia and neurons [50,51]. We assume that increased production of free fatty acids by demyelination leads to their elevated mitochondrial and peroxisomal degradation, followed by the increased formation of acetate and formate. We suggest that the quantities of newly generated acetate and formate bodies exceed the catabolic capacity of the brain cells and therefore lead to their accumulation in the CSF of our MS patients.
Alanine
A variety of data suggest that beta-alanine should be considered a small-molecule neurotransmitter that may function as an important carrier for ammonia transfer to complete brain glutamate-glutamine cycling. Alanine is a non-essential amino acid, crossing BBB by transport systems [19]. In vitro, both cortical and cerebral neurons show several-fold-higher de novo synthesis and release of alanine [53,54] confirming neuronal bonding of nitrogen. We found a significant increase in alanine CSF level in MS patients correlating with age, DD and disability score, which may show alanine participation in disease progress and duration. However, alanine may be a part of pyruvate recycling, where the changes of alanine with the age should be expected [55]. Therefore, a link between alanine and MS is only disputable.
Other unchanged metabolites
Creatine and creatinine serve as indices of one aspect of brain energy metabolism, the creatine-creatine phosphate shuttle. No changes in CSF levels of both metabolites in our study indicate that this part of energy metabolism is not affected in CIS (MS patients after the first clinical relapse) and in MS, or DEM patients.
Furthermore, we did not find an alteration in CSF levels of tyrosine and phenylalanine. In the brain tyrosine functions as a precursor of catecholamines (dopamine, norepinephrine, epinephrine). It was reported that the activity of neurotransmitter dopamine decreases in the CSF in MS [56]. However, we did not prove that.
Limitations
Although we have identified disease unique metabolite profiles, the future use of metabolite biomarkers in the clinical environment will require optimisation to improve accuracy. In our study, the accuracy of disease-unique metabolites profiles may be affected by a lower number of participants in the groups and heterogeneity of a disease course in both MS and non-MS diseases. Other limitation of this study could be the fact that we tested CSF in isolation, the samples were not coupled with serum of study participants. We did not test anti-MOG in all study participants due to technical problems. The test was not widely available in our country at the time of study beginning. Further studies may improve our knowledge about CSF biomarkers in demyelinating disorders.
Conclusion
In our work, glutamine, which correlated with EDSS, was the only metabolite capable of distinguishing between MS after the first clinical relapse or CIS and MS concerning other forms of the demyelinating brain diseases and controls. This work is the first one to show the predictive role of CSF glutamine in the early stages of MS, pinpointing an important role of the glutamate/glutamine cycle in MS pathogenesis. Another potential predictor of MS was citrate, as citrate CSF levels specified MS and CIS against DEM. However, it did not help to distinguish between early and later stages of MS. Citrate showed significant correlations with age, DD and EDSS in MS patients. The BCAA (acetate, acetone, pyruvate, formate, histidine) CSF levels are not significant predictors of MS.
Ethical statement
This study was performed following the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Ethics approval was performed by the Ethical committee of the Jessenius Faculty of Medicine in Martin, Comenius University (EK 1678/2015). Informed written consent was obtained from all study participants.
Acknowledgments
The study was supported by Grant VEGA 1/0301/19 andAPVV 15-0107 and BioMed Martin (ITMS 26220220187), co-funded from EU sources.
Disclosures
The authors declare they have no potential conflicts of interest concerning drugs, products or services used in the study.
Authors contribution
EB, LP, SS, EK – research, data collection, clinical assessment; EB, EK – study design, writing of the manuscript; EB – performance of 1H-NMRS, statistical analyses, collection of the data; DC, PH, SS, JL, EK – critical reading and correction of the manuscript.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Assist. Prof. Ema Kantorová, MD, PhD
Clinic of Neurology Jessenius Faculty of Medicine Comenius University in Bratislava and University Hospital Martin
Malá Hora 4A
036 01 Martin
Slovakia
e-mail: ema.kantorova@uniba.sk
Accepted for review: 9. 1. 2020
Accepted for print: 29. 4. 2020
Sources
1. Levin MC, Douglas JN, Meyers L et al. Neurodegeneration in multiple sclerosis involves multiple pathogenic mechanisms. Degener Neurol Neuromuscul Dis 2014 : 4; 49–63. doi: 10.2147/DNND.S54391.
2. Štourač P. Imunologická léčba roztroušené sklerózy mozkomíšní v klinických a zobrazovacích parametrech. Cesk Slov Neurol N 2012; 75/108 (4): 404–410.
3. Polman CH, Reingold SC, Banwell B et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69 (2): 292–302. doi: 10.1002/ana.22366.
4. Deisenhammer F, Zetterberg H, Fitzner B et al. The cerebrospinal fluid in multiple sclerosis. Front Immunol 2019; 10 : 726. doi: 10.3389/fimmu.2019.00726.
5. Hnilicová P, Kantorová E, Poláček H et al. Altered hypothalamic metabolism in early multiple sclerosis – MR spectroscopy study. J Neurol Sci 2019; 407 : 116458. doi: 10.1016/j.jns.2019.116458.
6. Sladkova V, Mareš J, Lubenova B et al. Degenerative and inflammatory markers in the cerebrospinal fluid of multiple sclerosis patients with relapsing-remitting course of disease and after clinical isolated syndrome. Neurol Res 2011 33 (4): 415–420. doi: 10.1179/016164110X12816242542535.
7. Lutz NW, Viola A, Malikova I et al. Inflammatory multiple sclerosis plaques generate characteristic metabolic profiles in cerebrospinal fluid. PLos One 2007; 2 (7): e595. doi: 10.1371/journal.pone.0000595.
8. French CD, Wiloughby RE, Wong Sj et al. NMR metabolomics of cerebrospinal fluid differentiates inflammatory diseases of the central nervous system. PLOS Negl Trop Dis 2018; 12 (12): e0007045. doi: 10.1371/journal.pntd.0007045.
9. Kim HH, Jeong IH, Hyun JS et al. Metabolomic profiling of CSF in multiple sclerosis and neuromyelitis optica spectrum disorder by nuclear magnetic resonance. PLoS One 2017; 12 (7): e0181758. doi: 10.1371/journal.pone.0181758.
10. Aasly J, Garseth M, Sonnewald U et al. Cerebrospinal fluid lactate and glutamine are reduced in multiple sclerosis. Acta Neurol Scand 1997; 95 (1): 9–12. doi: 10.1111/j.1600-0404.1997.tb00060.x.
11. Herman S, Åkerfeldt T, Spjuth O et al. Biochemical differences in cerebrospinal fluid between secondary progressive and relapsing-remitting multiple sclerosis. Cells 2019; 8 (2): 84. doi: 10.3390/cells8020084.
12. Cruz T, Balayssac S, Gilard V et al. 1H NMR Analysis of cerebrospinal fluid from Alzheimer’s disease patients: an example of a possible misinterpretation due to non - adjustment of pH. Metabolites 2014; 4 (1): 114–128. doi: 10.3390/metabo4010115.
13. Lutz NW, Cozzone PJ. Metabolomic profiling in multiple sclerosis and other disorders by quantitative analysis of cerebrospinal fluid using nuclear magnetic resonance spectroscopy. Curr Pharm Biotechnol 2011; 12 (7): 1016–1025. doi: 10.2174/138920111795909122.
14. Barkhof F, Filippi M, Miller D et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 1997; 120 (Pt 11): 2059–2069. doi: 10.1093/brain/120.11.2059.
15. Albrecht J, Sonnewald U, Waagepetersen HS et al. Glutamine in the central nervous system: function and dysfunction. Front Biosci 2007; 12 : 332–343. doi: 10.2741/2067.
16. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 2000; 130 (4S Suppl): 1007S–1015S. doi: 10.1093/jn/130.4.1007S.
17. Barkhatova VP, Zavalishin IA, Askarova LS et al. Changes in neurotransmitters in multiple sclerosis. Neurosci Behav Physiol 1998 : 28 (4): 341–344. doi: 10.1007/ BF02464784.
18. Sarchielli P, Greco L, Floridi A et al. Excitatory amino acids and multiple sclerosis: evidence from cerebrospinal fluid. Arch Neurol 2003; 60 (8): 1082–1088. doi: 10.1001/archneur.60.8.1082.
19. Hawkins RA, O‘Kane RL, Vina JR. Structure of the blood–brain barrier and its role in the trans-port of amino acids. J Nutr 2006; 136 (1 Suppl): 218S–226S. doi: 10.1093/jn/136.1.218S.
20. Lee WJ, Hawkins RA, Vina JR et al. Glutamine transport by the blood-brain barrier: a possible mechanism for nitrogen removal. Am J Physiol 1998; 274 (4): C1101–C1104. doi: 10.1152/ajpcell.1998.274.4.C1101.
21. Xu GY, McAdoo DJ, Hughes MG et al. Considerations in the determination by microdialysis of resting extracellular amino acid concentrations and release upon spinal cord injury. Neuroscience 1998; 86 (3): 1011–1021. doi: 10.1016/s0306-4522 (98) 00063-3.
22. Wang Y, Zhu M, Han J et al. Blood brain barrier perrmeability could be a biomarker to predict severity of neuromyelitis optica spectrum disorders: a retrospective analysis. Front Neurol 2018; 9 : 648. doi: 10.3389/fneur.2018.00648.
23. Carr EL, Kelman A, Wu GS et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol 2010; 185 (2): 1037–1044. doi: 10.4049/jimmunol.0903586.
24. Zhou Y, Danboll NC. Glutamate as a neurotransmitter in the healthy brain. J Neural Transm (Vienna) 2014; 121 (8): 799–817. doi: 10.1007/s00702-014-1180-8.
25. Prineas J. Pathology of the early lesion in multiple sclerosis. Hum Pathol 1975; 6 (5): 531–554. doi: 10.1016/s0046-8177 (75) 80040-2.
26. Medera C, Vargas-Lopes C, Brandai CO et al. Elevated glutamate and glutamine levels in the cerebrospinal fluid of patients with probable alzheimer‘s disease and depression. Front Psychiatry 2018; 9 : 561. doi: 10.3389/fpsyt.2018.00561.
27. Camu W, Biliard M, Maldy-Moulinier M. Fasting plasma and CSF amino acid levels in amyotrophic lateral sclerosis: a subtype analysis. Acta Neurol Scand 1993; 88 (1): 51–55. doi: 10.1111/j.1600-0404.1993.tb04186.x.
28. Yudkoff M. Interactions in the metabolism of glutamate and the branched-chain amino acids and ketoccids in the CNS. Neurochem Res 2017; 42 (1): 10–18. doi: 10.1007/s11064-016-2057-z.
29. Murin R, Mohammadi G, Leibfritz D et al. Glial metabolism of isoleucine. Neurochem Res 2009; 34 (7): 1195–1203. doi: 10.1007/s11064-008-9895-2.
30. Murin R, Hamprecht B. Metabolic and regulatory roles of leucine in neural cells. NeurochemRes 2008; 33 (2): 279–284. doi: 10.1007/s11064-007-9444-4.
31. Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab 2012; 32 (7): 1107–1138. doi: 10.1038/jcbfm.2011.175.
32. De Simone R, Vissicchio F, Mingarelli C et al. Branched-chain amino acids influence the immune properties of microglial cells and their responsiveness to pro-inflammatory signals. Biochim Biophys Acta 2013; 1832 (5): 650–659. doi: 10.1016/j.bbadis.2013.02.001.
33. O’Brien JS, Sampson EL. Fatty acid and fatty aldehyde composition of the major brain lipids in normal human gray matter, white matter, and myelin. J Lipid Res 1965; 6 (4): 545–551.
34. Sonnewald U, Westrgaard N, Unsgard G et al. First direct demonstration of a preferential release of citrate from astrocytes using [13C] NMR spectroscopy of cultured neurons and astrocytes. Neurosci Lett 1991; 128 (2): 235–239. doi: 10.1016/0304-3940 (91) 90268-x.
35. Westergaard N, Sonnewald U, Unsgard G et al. Uptake, release, and metabolism of citrate in neurons and astrocytes in primary cultures. J Neurochem 1994; 62 (5): 1727–1733. doi: 10.1046/j.1471-4159.1994.62051727.x.
36. Westergaard N, Banke T, Sonnewald U et al. Citrate modulates the regulation by Zn2+ of N-methyl-D-aspartate receptor-mediated channel current and neurotransmitter release. Proc Natl Acad Sci U S A 1995; 92 (8): 3367–3370. doi: 10.1073/pnas.92.8.3367.
37. Westergaard N, Waagepetersen HS, Belhage B et al.Citrate, a ubiquitous key metabolite with regulatory function in the CNS. Neurochem Res 2017; 42 (6): 1583–1588. doi: 10.1007/s11064-016-2159-7.
38. Reinkee SN, Broadhurst DI, Sykes BD et al. Metabolomic profiling in multiple sclerosis: insights into biomarkers and pathogenesis. Mult Scler J 2014; 20 (10): 1396–1400. doi: 10.1177/1352458513516528.
39. Simone IL, Federico F, Trojano M et al. High resolution proton MR spectroscopy of cerebrospinal fluid in MS patients. Comparison with biochemical changes in demyelinating plaques. J Neurol Sci 1996; 144 (1–2): 182–190. doi: 10.1016/s0022-510x (96) 00224-9.
40. Cocco E, Murgia F, Lorefice L et.al. 1H-NMR analysis provides a metabolomic profile of patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2015; 3 (1): e185. doi: 10.1212/NXI.0000000000000185.
41. Mao P, Reddy PH. Is multiple sclerosis a mitochondrial disease? Biochim Biophys Acta 2010; 1802 (1): 66–79. doi: 10.1016/j.bbadis.2009.07.002.
42. Kallweit U, Aritake K, Bassetti CL et al. Elevated CSF histamine levels in multiple sclerosis patients. Fluids Barriers CNS 2013; 10 : 19. doi: 10.1186/2045-8118-10-19.
43. Tuomisto L, Kilpelainen H, Riekkinen P. Histamine and histamine-N-methyltransferase in the CSF of patients with multiple sclerosis. Agents Actions 1983; 13 (2–3): 255–257. doi: 10.1007/BF01967346.
44. Polyzoidis S, Koletsa T, Panagiotidou S et al. Mast cells in meningiomas and brain inflammation. J Neuroinflammation 2015; 12 : 170. doi: 10.1186/s12974-015-0388-3.
45. Sinclair AJ, Viant MR, Ball AK et al. NMR-based metabolomic analysis of cerebrospinal fluid and serum in neurological diseases – a diagnostic tool? NMR Biomed 2010; 23 (2): 123–132. doi: 10.1002/nbm.1428.
46. Ronowska A, Szutowicz A, Bielarczyk H et al. The regulatory effects of acetyl - CoA distribution in the healthy and diseased brain. Front Cell Neurosci 2018; 12 : 169. doi: 10.3389/fncel.2018.00169.
47. Ariyannur PS, Moffett JR, Madhavarao CN et al. Nuclear-cytoplasmic localization of acetyl coenzyme a synthetase-1 in the rat brain. J Comp Neurol 2011; 518 (15): 2952–2977. doi: 10.1002/cne.22373.
48. Van Veldhoven PP. Biochemistry and genetics of inherited disorders of peroxisomal fatty acids metabolism. J Lipid Res 2010; 51 (10): 2863–2895. doi: 10.1194/jlr.R005959.
49. Bao XR, Ong SE, Goldberger O et al. Mitochondrial dysfunction remodels one - carbon metabolism in human cells. Elife 2016; 5: e10575. doi: 10.7554/eLife.10575.
50. Nicklas WJ, Clarke DD, Berl S. Decarboxylation studies of glutamate, gltamine and aspartate from brain labeled with [1-14C] acetate, [L-U-14C] aspartate and [L-U - 14C glutamate]. J Neurochem 1969 : 16 (4): 549–558. doi: 10.1111/j.1471-4159.1969.tb06854.x.
51. Badar-Goffer RS, Bachelard HS, Morris PG. Cerebral metabolism of acetate and glucose studied by 13C-n. m.r. spectroscopy. A technique for investigating metabolic compartmentation in the brain. Biochem J 1990; 266 (1): 133–139. doi: 10.1042/bj2660133.
52. Hawkins RA. The blood-brain barrier and glutamate. Am J Clin Nutr 2009; 90 (3): 867S–874S. doi: 10.3945/ajcn.2009.27462BB.
53. Waagepetersen HS, Sonnewald U, Larsson OM et al.A possible role of alanine for ammonia transfer between astrocytes and glutamatergic neurons. J Neurochem 2000; 75 (2): 471–479. doi: 10.1046/j.1471-4159.2000.0750471.x.
54. Zwingmann C, Richter-Landsberg C, Brand A et al.NMR spectroscopic study on the metabolic fate of [3 - (13) C]alanine in astrocytes, neurons, and cocultures: implications for glianeuron interactions in neurotransmitter metabolism. Glia 2000; 32 (3): 286–303. doi: 10.1002/1098-1136 (200012) 32 : 3<286:: aid-glia80>3.0.co; 2-p.
55. Jiang T, Cadenas E. Astrocytic metabolic and inflammatory changes as a function of age. Aging cell 2014; 13 (6): 1059–1067. doi: 10.1111/acel.12268.
56. Markianos M, Koutsis G, Evangelopoulos ME et al. Relationship of CSF neurotransmitter metabolitte levels to disease severity and disability in multiple sclerosis. J Neurochem 2009; 108 (1): 158–164. doi: 10.1111/j.1471-4159.2008.05750.x.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2020 Issue 3
-
All articles in this issue
- Primary progressive aphasia
- Are we close to targeted treatments for Huntington’s disease? YES
- Are we close to targeted treatments for Huntington’s disease? NO
- Are we close to targeted treatments for Huntington’s disease? COMMENT
- Cognitive disorders in children with epilepsy
- Autoimmune encephalitis with negative anti-neuronal antibodies – clinical characteristics and available methods of antibody detection
- APOE and BDNF as genetic risk markers for predicting the onset and development of cognitive deficits due to Alzheimer’s disease
- Role of novel laboratory techniques in Niemann-Pick type C disease diagnostics
- Headaches in pregnancy
- Occurrence and risk factors of unprovoked epileptic seizures in ischaemic stroke patients
- Validation of DYsphagia in MUltiple Sclerosis questionnaire – Czech version of DYMUS
- Effect of a neuropalliative care intervention on quality of life in patients with progressive neurological disease – interventional study
- Characteristics of a cohort of boys with Duchenne and Becker muscular dystrophies – a study from a single neuromuscular centre
- Epidural application of steroids Part 2 – The quality of life of patiens before application
- Glioblastoma grade IV – long-term survival
- Stanovisko redakční rady k diskuzi o úrovni a směřování Cesk Slov Neurol N
- Erratum
- Informace vedoucího redaktora
- Recenze
- Impact of dementia on the trajectories of quality of life in older adults
- Magnetic resonance spectroscopy metabolomics of cerebrospinal fluid in patients with multiple sclerosis, clinically isolated syndrome, other inflammatory brain diseases and controls
- Focal epileptic seizure in a young female from South Korea
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Glioblastoma grade IV – long-term survival
- Primary progressive aphasia
- Headaches in pregnancy
- Cognitive disorders in children with epilepsy
