Flora characteristics and risk factors of lower respiratory tract infections in patients with severe craniocerebral trauma in the intensive care unit
Vlastnosti mikroflóry a rizikové faktory infekcí dolních cest dýchacích u pacientů se závažným kraniocerebrálním poraněním na jednotce intenzivní péče
Cíl: Cílem této studie bylo prozkoumat vlastnosti mikroflóry a rizikové faktory infekcí dolních cest dýchacích u pacientů se závažným kraniocerebrálním poraněním na jednotce intenzivní péče. Soubor pacientů a metody: Byla shromážděna klinická data 457 vhodných pacientů hospitalizovaných od srpna 2015 do března 2020 a byly zaznamenány výskyt infekcí, distribuce patogenů a výsledky testů citlivosti na antimikrobiální léčiva. Byly analyzovány příslušné rizikové faktory. Výsledky: Z celkového počtu 457 mělo 150 pacientů infekce dolních cest dýchacích. Po kultivaci vzorků sputa bylo izolováno celkem 78 (52,00 %) kmenů grampozitivních bakterií, 65 (43,33 %) kmenů gramnegativních bakterií a 7 (4,67 %) kmenů kvasinek a plísní. Mezi nejčastějšími gramnegativními bakteriemi bylo zachyceno více multirezistentních nebo plně rezistentních kmenů. Grampozitivní bakterie vykazovaly 100% citlivost vůči vankomycinu and teikoplaninu a pouze Staphylococcus haemolyticus byl plně rezistentní vůči erythromycinu. Mezi nezávislé rizikové faktory patřily věk, doba trvání kómatu, tracheální intubace nebo incize, použití umělé plicní ventilace a podávání antibiotik (p < 0,05). Závěr: Nejčastější patogeny vyvolávající infekce dolních cest dýchacích u pacientů se závažným kraniocerebrálním poraněním na jednotce intenzivní péče vykazují rezistenci na široké spektrum antimikrobiálních léčiv a vysokou míru rezistence. Je nutné zajistit operace v aseptickém prostředí, striktně zabránit zkříženým infekcím a zlepšit imunitní funkce pacientů, čímž dojde ke zlepšení terapeutického účinku.
Klíčová slova:
rizikový faktor – jednotka intenzivní péče – závažné kraniocerebrální poranění – infekce dolních cest dýchacích – mikroflóra
Authors:
M. Zhang; D. Zhi; J. Lin; P. Liu; Y. Wang; M. Duan
Authors‘ workplace:
Department of Critical Care Medicine, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Published in:
Cesk Slov Neurol N 2022; 85(5): 375-381
Category:
Original Paper
doi:
https://doi.org/10.48095/cccsnn2022375
Overview
Aim: The aim of the study was to explore the flora characteristics and risk factors of lower respiratory tract infections in patients with severe craniocerebral trauma in intensive care unit. Patients and methods: The clinical data of 457 eligible patients hospitalized from August 2015 to March 2020 were collected, and incidence rate of infections, pathogen distribution and drug sensitivity test results were recorded. Related risk factors were analyzed. Results: Out of the 457 patients, 150 had lower respiratory tract infections. A total of 78 (52.00%) strains of Gram-positive bacteria, 65 (43.33%) strains of Gram-negative bacteria, and 7 (4.67%) strains of fungi were isolated through sputum culture. Multi-drug resistant and fully resistant strains in main Gram-negative bacteria had higher detection rates. Gram-positive bacteria were 100% sensitive to vancomycin and teicoplanin and only Staphylococcus haemolyticus was fully resistant to erythromycin. Age, duration of coma, tracheal intubation or incision, use of ventilators, and use of antibiotics were independent risk factors (P < 0.05). Conclusion: The main pathogens for lower respiratory tract infections in patients with severe craniocerebral trauma in intensive care unit have extensive drug resistance and high resistance rates. It is necessary to ensure aseptic operations, strictly prevent cross infections, and improve the immune function of patients, thereby improving the therapeutic effect.
Keywords:
risk factor – intensive care unit – severe craniocerebral trauma – lower respiratory tract infection – flora
Introduction
Severe craniocerebral trauma, a common critical neurosurgical disease in the intensive care unit (ICU), progresses rapidly, and it is extremely likely to cause the death of patients if not treated promptly. Clinically, the common invasive therapies include tracheal intubation or incision and use of ventilators. However, patients in the ICU are easily afflicted with bacterial infections, the most common of which are lower respiratory tract infections due to the critical disease condition-caused decline in immunity together with invasive treatments and long-term use of high-dose broad-spectrum antibacterial drugs, diuretics and hormones [1]. Notably, bacterial drug resistance has become increasingly prominent due to massive use of antibacterial drugs [2]. In recent years, lower respiratory tract infections are often considered as important risk factors for the death of patients with severe craniocerebral trauma [3]. Therefore, it is of great significance to explore the pathogen distribution, clinical characteristics, and risk factors of lower respiratory tract infections in patients with severe craniocerebral trauma in the ICU for the prevention and control of bacterial infections and the improvement of rescuing success rate and survival quality of critical patients.
The aim of this study was to investigate the flora characteristics and risk factors of lower respiratory tract infections, and to provide bases for the clinical prevention, control and treatment of lower respiratory tract infections in patients with severe craniocerebral infections in the ICU.
Patients and methods
Baseline clinical data
In our retrospective study, a total of 457 patients with severe craniocerebral trauma hospitalized in the ICU of our hospital from August 2015 to March 2020 were enrolled as subjects. Inclusion criteria were: Glasgow Coma Scale score of 3–8 points; availability of complete clinical data and informed consent voluntarily signed by relatives. Exclusion criteria were: history of combined injury, multiple injuries and craniocerebral trauma as confirmed by physical examination and head CT; history of acute and chronic inflammation, diabetes, abnormal renal function, pulmonary diseases and pulmonary contusion before trauma; symptoms and signs of lower respiratory tract infections within 48 h after admission; food consumption within 4–6 h prior to admission; the use of glucocorticoid, sugar-containing drugs, anticoagulants or the drugs affecting fibrinolytic activity before admission.
Among the 457 patients with severe craniocerebral trauma, 318 were males, and they were 30–78 years old with a mean of 43.52 ± 5.61 years. There were 99 cases of injuries due to falling on the ground, 175 cases of high fall injuries, 61 cases of blunt trauma injuries, 122 cases of traffic accident injuries, 96 cases of brain-stem injuries, 61 cases of epidural hematoma, 128 cases of subdural hemorrhage, 82 cases of subarachnoid hemorrhage, and 90 cases of intracerebral hematoma.
Diagnostic criteria for lower respiratory tract infection
According to the Guidelines for the Management of Adult Lower Respiratory Tract Infections [4], patients had such clinical manifestations as cough, cough producing purulent sputum and pulmonary moist rales, and any of the following conditions: body temperature > 37.5 °C; inflammatory infiltrative lesions in the lungs as shown in chest imaging examination; and positive bronchial secretion culture results or a white blood cell count > 12.0×109/mL as indicated in laboratory tests.
Isolation and identification of bacteria
Clinical medical staff collected specimens with reference to the National Guide to Clinical Laboratory Procedures (4th Ed.) [5]. First, sputum was collected into an aseptic container using a closed endotracheal tube and timely sent to the Microbiological Laboratory for pathogenic detection. The specimens sent for detection were considered to meet quality control criteria, if it was microscopically observed that the ratio of multinuclear leukocytes to squamous epithelial cells in the sputum specimens was lower than 2.5, or the count of multinuclear leukocytes was not lower than 25 and that of squamous epithelial cells was not higher than 10 in each low-power field of view. The sticky sputum needed to be trypsinized first. Then, the sputum specimens were inoculated onto a blood agar plate, a chocolate agar plate or a MacConkey agar plate on an aseptic operator’s station and cultured in an incubator at 37 °C for 24 h. Following growth of bacterial strains, a single bacterial colony was picked up for Gram staining. Based on the staining results, the types of bacteria were identified using the VITEK 2 Compact automatic microorganism identification and drug sensitivity analyzer (BioMérieux, Marcy - l’Étoile, France) and its equipped reagents strictly in accordance with operation specifications.
Drug susceptibility test
Routine drug susceptibility test was conducted for the isolated and cultured pathogens using the above-mentioned automatic microorganism identification and drug sensitivity analyzer and its equipped drug sensitivity cards, and the test was repeated 3 times to ensure the accuracy of the results. Staphylococcus aureus (ATCC25923), Pseudomonas aeruginosa (ATCC27853), Acinetobacter baumannii (ATCC19606), Escherichia coli (ATCC25922), Enterococcus fae - calis (ATCC29212), Klebsiella pneumoniae (ATCC700603) and Candida albicans (ATCC 14053) (the National Center for Clinical Laboratories) were taken as quality control strains. Finally, laboratory test results were assessed according to the criteria formulated by the USA Clinical & Laboratory Standards Institute [6].
Statistical analysis
All data were statistically analyzed using SPSS 16.0 software (IBM, Armonk, NY, USA). The numerical data were expressed as case (%) and subjected to the Chi-square test. The quantitative data were represented as mean ± standard deviation and subjected to the independent t-test. The risk factors for the occurrence of lower respiratory tract infections were analyzed by using the logistic regression model. P < 0.05 was considered statistically significant.
Results
Lower respiratory tract infection rate and distribution of pathogenic bacteria
There were 150 cases of lower respiratory tract infections among the 457 enrolled patients with severe craniocerebral trauma, and the infection rate was 32.85%. A total of 78 (52.00%) strains of Gram-negative bacteria, dominated by P. aeruginosa, A. baumannii and K. pneumoniae, 65 (43.33%) strains of Gram-positive bacteria, including S. aureus, S. haemolyticus and E. faecalis, and 7 (4.67%) strains of fungi, including C. albicans were isolated and identified through the sputum culture in the 150 cases (Tab. 1).
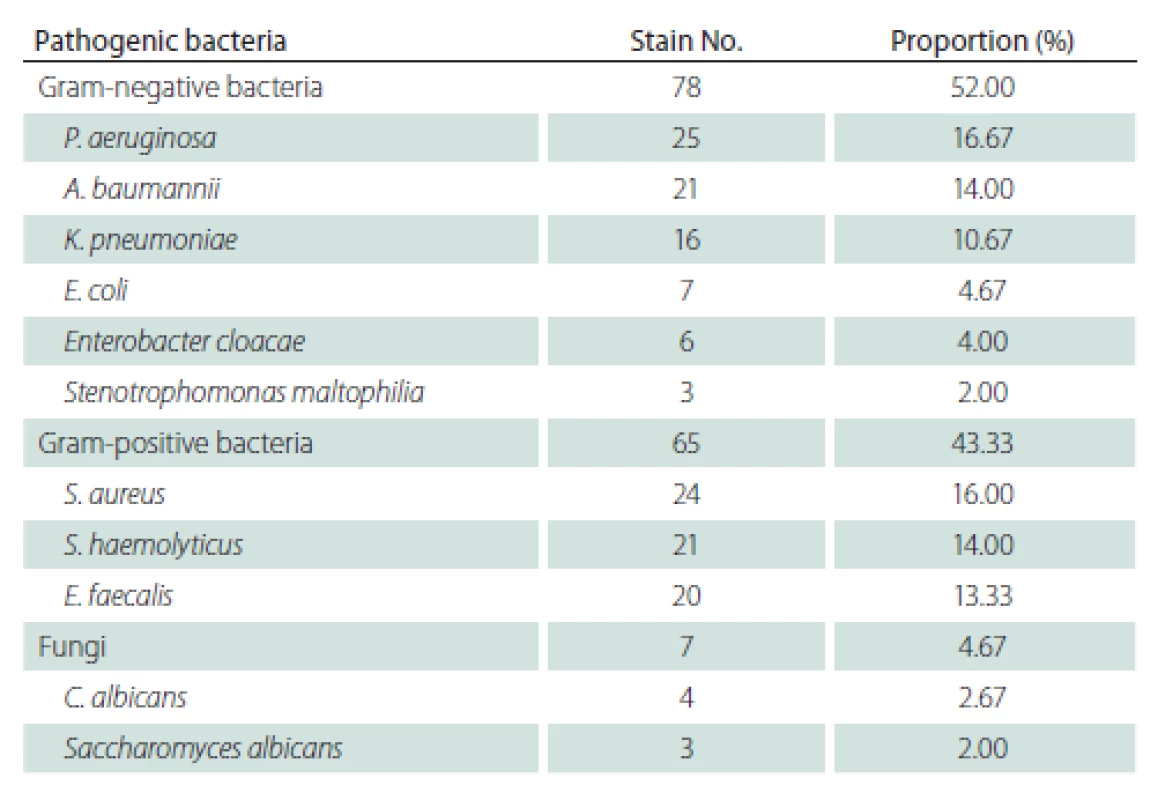
Drug susceptibility of main Gram-negative bacteria
Gram-negative bacteria, among the top 3 in terms of composition proportion, were subjected to a drug sensitivity test, and it was found that multi-drug resistant strains and fully resistant strains had higher detection rates. P. aeruginosa exhibited 100% resistance to ceftriaxone, ampicillin, ampicillin/sulbactam, and sulfamethoxazole/trimethoprim, 96% resistance to cefazolin, and 88% resistance to cefotetan, whereas it was 100% sensitive to tobramycin, amikacin, and ertapenem and < 20% resistant to other antimicrobial agents. A. baumannii was 100% resistant to cefazolin, cefotetan, amikacin and ampicillin and 100% sensitive to ertapenem, with varying rates of resistance to other antibiotics. K. pneumoniae was fully resistant only to ampicillin, with no resistance to tobramycin, amikacin and ertapenem and different degrees of resistance to other antibacterial drugs (Tab. 2).
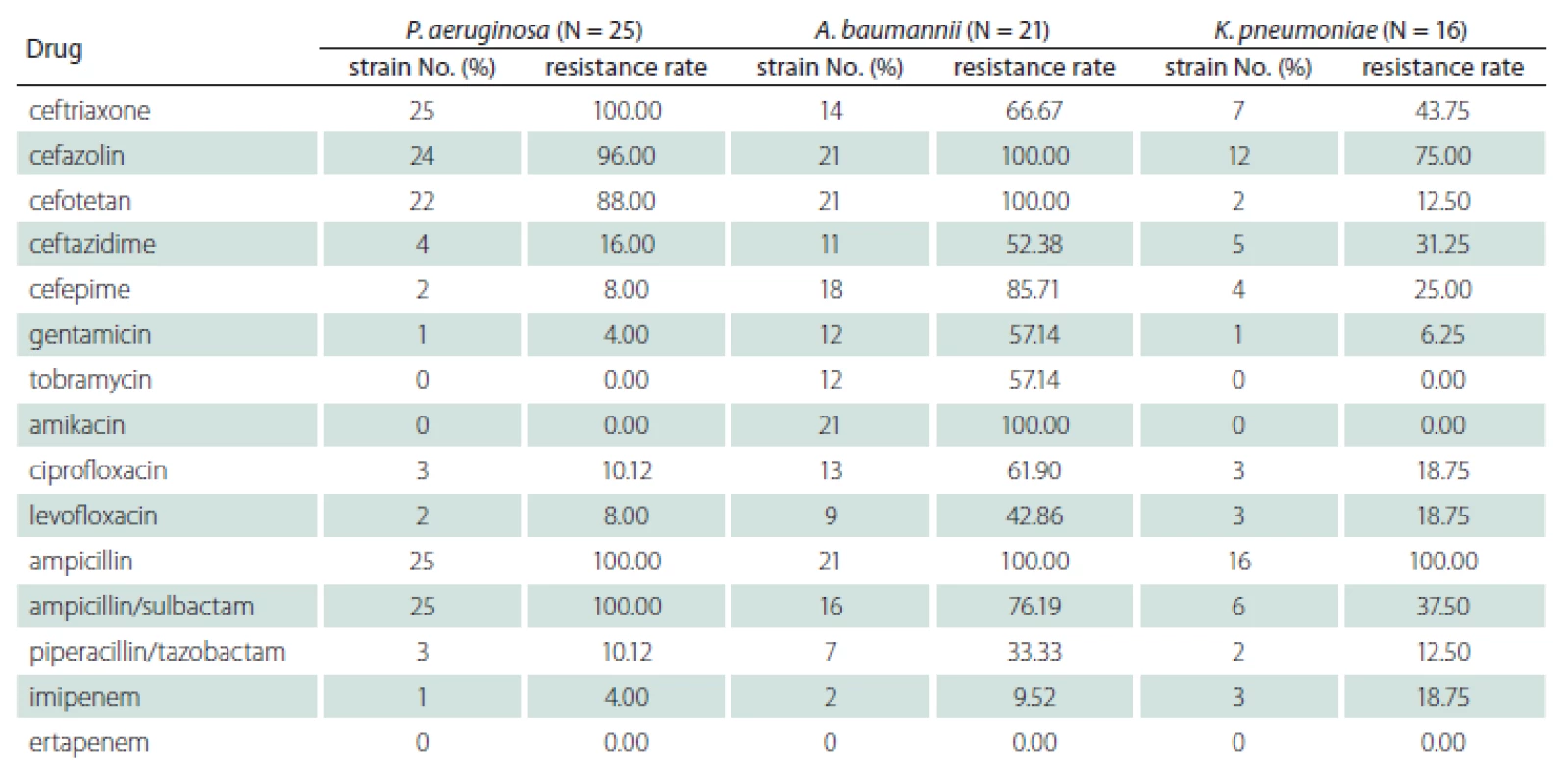
Drug susceptibility of Gram-positive bacteria
Based on the drug sensitivity test results of Gram-positive bacteria, S. aureus showed the highest rate of resistance to penicillin G (91.67%) and 100% sensitivity to vancomycin, teicoplanin, and linezolid. S. haemolyticus was fully resistant to erythromycin, 95.24% resistant to penicillin G, and 90.48% resistant to gentamicin, with 100% sensitivity to vancomycin, teicoplanin and linezolid. The resistance rate of E. faecalis to penicillin G was 95%, followed by 90% resistance to oxacillin, 55% resistance to levofloxacin, and < 50% resistance to other antibiotics, and it was 100% sensitive to vancomycin and teicoplanin (Tab. 3).
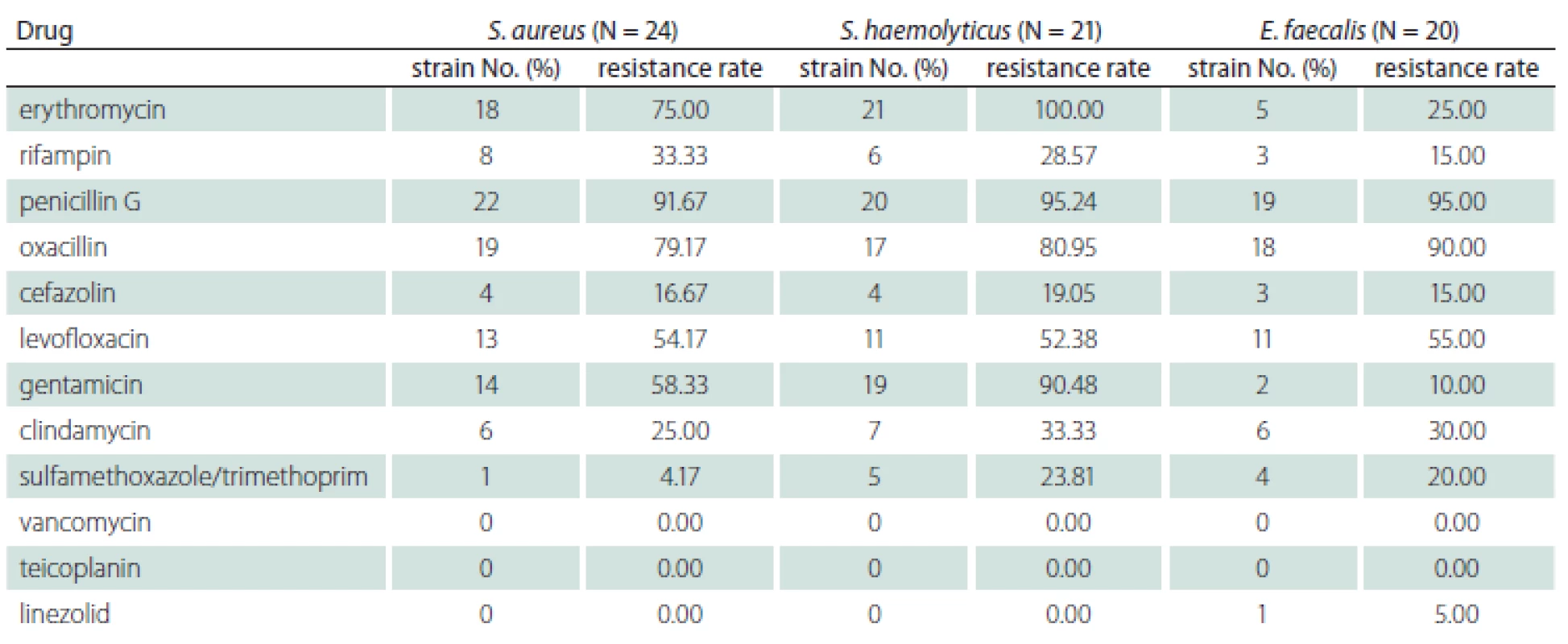
Univariate analysis results of factors affecting lower respiratory tract infections
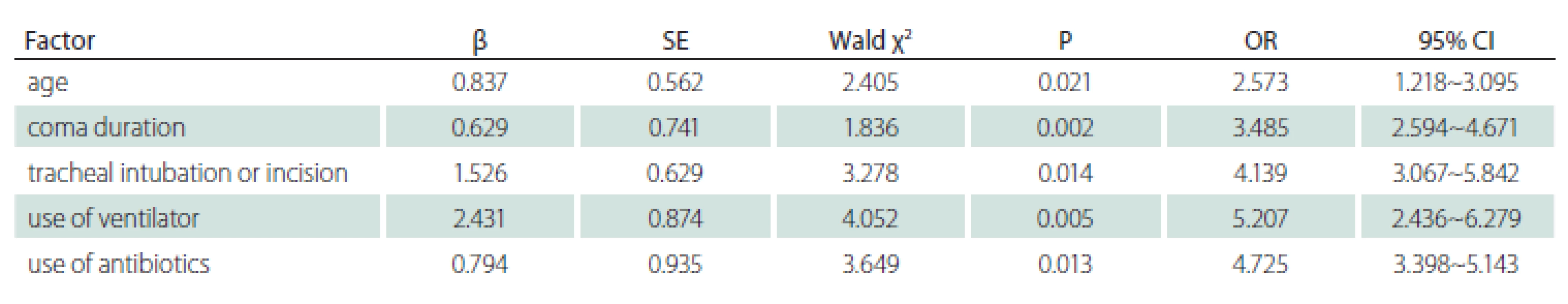
According to the presence or absence of lower respiratory tract infections, patients with severe craniocerebral trauma were allocated into the infection group and non-infection group, and it was discovered that the two groups of patients were not statistically significantly different regarding sex, causes of injuries, injured site, smoking history, nasal feeding, sputum aspiration and indwelling of venous catheters (P > 0.05). Compared with the non-infection group, the infection group patients exhibited a higher age, longer duration of coma, and more cases of tracheal intubation or incision, use of ventilators and use of antibiotics, displaying statistically significant differences (P < 0.05) (Tab. 4).
Multivariate logistic regression analysis results of factors affecting lower respiratory tract infections
Logistic regression analysis was conducted on the statistically significant variables in the univariate analysis as independent variables and the development of lower respiratory tract infections as the dependent variable. According to the results, patients age, duration of coma, tracheal intubation or incision, use of ventilators and use of antibiotics were the risk factors for the occurrence of lower respiratory tract infections in patients with severe craniocerebral trauma in the ICU (P < 0.05) (Tab. 5).
Discussion
Since patients with severe craniocerebral trauma in the ICU are seriously injured and suffer from complications such as shock, coma, combined chest and abdominal injuries and multiple fractures, their immune function is compromised. Besides, clinical rescue approaches such as invasive operations and massive use of antibiotics and immunosuppressors are extremely likely to induce in-hospital infections in patients, of which the most common and severe are lower respiratory tract infections. These mainly affect the important organs maintaining the ventilation of human body, including the trachea, bronchi and lungs, so they can aggravate craniocerebral trauma and worsen the prognosis.
In the present study, among the 457 enrollees with severe craniocerebral infections, 150 had lower respiratory tract infections, with an infection rate of 32.85%. It was found through the culture of sputum specimens that Gram-negative bacteria were the major pathogens causing lower respiratory tract infections in patients with severe craniocerebral trauma, consistent with numerous literature reports worldwide [7]. Additionally, many Gram-positive bacteria and few fungi were observed, which suggests the complex distribution of the pathogens responsible for lower respiratory tract infections in the Infection ICU. Among Gram-negative bacteria, P. aeruginosa, A. baumannii and K. pneumoniae were among the top 3 in terms of the composition proportion, the detected Gram-positive bacteria included S. aureus, S. haemolyticus, and E. faecalis, and C. albicans was the detected fungus. The drug sensitivity test results revealed that there were more multi-drug resistant strains and fully resistant strains in the major Gram-positive bacteria, whereas Gram-positive bacteria were 100% sensitive to vancomycin and teicoplanin, of which only S. haemolyticus was fully resistant to erythromycin. Moreover, different pathogens exhibited relatively extensive drug resistance, and their resistance to the same antibiotic varied to a certain extent. The distribution and drug resistance of pathogens in lower respiratory tract infections were not completely consistent with previous literature [8]. It is thus inferred that dominant resistant strains were altered due to the smaller sample size in the present study, differences in the antibiotic types and doses used in regions or hospitals, and the drug resistance mechanisms of different flora. Therefore, antibiotics to which pathogens are sensitive should be chosen carefully and properly used based on the results of the pathogen test and drug susceptibility test in the clinical treatment of lower respiratory tract infections in patients with severe craniocerebral trauma in the ICU.
Invasive operations and use of antibiotics have been reported to be the risk factors for lower respiratory tract infections in patients with craniocerebral trauma-induced coma [9]. Additionally, body temperature, blood glucose, duration of coma, duration of hospital stay, indwelling time of gastric tube, tracheal incision and use of ventilators are the risk factors for lower respiratory tract infections in patients with severe craniocerebral trauma in the ICU [10]. According to the results of this study, the development of lower respiratory tract infections in patients with severe craniocerebral trauma in the ICU was closely related to patients age, duration of coma, tracheal intubation or incision, use of ventilators and use of antibiotics. This finding is consistent with the above-mentioned study reports. As age increases, the organ functions and body immunity of patients will decline together with atrophy of respiratory mucosa and decreased ciliary motility, which weakens cough, expectoration and other normal physiological functions. Moreover, patients with severe craniocerebral trauma are in a coma, so the secretions in the lower respiratory tract fail to be normally discharged, thereby triggering lower airway obstruction. Ultimately, infections are induced due to retention of massive inhaled pathogens in the lower respiratory tract [11]. Additionally, coma greatly weakens the swallowing function of patients with severe craniocerebral trauma, and intracranial hypertension is extremely likely to induce vomiting, thereby resulting in accidental aspiration. Gastric contents reversely flow into the respiratory tract and further worsen its obstruction and mucosal damage, thus heightening the risk of infections [12]. Tracheal intubation or incision for opening the airway in patients with severe craniocerebral trauma seriously destroys the filtration, humidification and thermoregulation functions of the respiratory tract and other natural protective barriers. The mucosal blood flow is altered since the tracheal wall continues to be compressed by the tube, and the high-pressure air bag blocks the blood between tracheal cartilages and impairs the defense function of the respiratory system. Besides, frequent negative-pressure suction can also damage respiratory mucosa, and pathogens in the air and below the pharynx adhere to the respiratory tract where they are colonized, thereby inducing infections [13]. Long-term use of ventilators can cause respiratory mucosal damage as well. In particular, the condensed water in the tube can contribute to massive multiplication of bacteria, and moving the tube or changing the positions of patients can extremely easily allow the condensate with bacteria to enter the lower respiratory tract, leading to infections. Therefore, ventilators should be strictly disinfected [14]. Additionally, long-time use of antibiotics tends to make strains drug-resistant, thereby giving rise to flora disorder, and it can suppress the normal immune response in organisms to a certain degree, ultimately inducing infections [15].
In summary, the major pathogens for lower respiratory tract infections in patients with severe craniocerebral trauma in the ICU have high drug resistance rates and extensive drug resistance, so antibiotics should be properly used for the treatment based on clinical trials to reduce bacterial resistance. Moreover, measures should be taken to ensure aseptic operations, strictly prevent cross infections, and improve the immune function of patients, thereby improving the efficacy. Based on the etiological characteristics and risk factors of lower respiratory tract infections in this study, it is recommended that the following prevention measures should be applied in patients with severe craniocerebral trauma in the ICU. The type and drug resistance of pathogens should be promptly identified for many times, and targeted medication should be initiated based on the test results if the conventional empirical treatments have poor efficacy. The wards need to be kept clean, and terminal disinfection should be regularly performed to strictly prevent cross infection if appropriate. Invasive operations should be completed aseptically, and patients should be cared for well. Once it is ensured that a primary disease is effectively treated, nutritional support treatment should be carried out to strengthen the immune functions of patients, especially the elderly.
Ethical aspects
The entire study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 2004 and 2008). This study was conducted upon the review and approval of the Medical Ethics Committee of Beijing Friendship Hospital, Beijing, China on August 4, 2015 (approval number: 2020 P2 065P01).
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Meili Duan
Department of Critical Care Medicine
Beijing Friendship Hospital
Capital Medical University
No. 95 Yong‘an Road
Xicheng District
Beijing 100050
China
e-mail: uc82160unb31@163.com
Accepted for review: 23. 12. 2021
Accepted for print: 28. 7. 2022
Sources
1. Wunderink RG. POINT: Should inhaled antibiotic therapy be used routinely for the treatment of bacterial lower respiratory tract infections in the ICU setting? Yes. Chest 2017; 151 (4): 737–739. doi: 10.1016/j.chest.2016.11.006.
2. Liu H, Liu B, Zheng F et al. Distribution of pathogenic bacteria in lower respiratory tract infection in lung cancer patients after chemotherapy and analysis of integron resistance genes in respiratory tract isolates of uninfected patients. J Thorac Dis 2020; 12 (8): 4216–4223. doi: 10.21037/jtd-20-928.
3. Figueroa L, Laffaye F. Early use of continuous positive airway pressure in the treatment of moderate to severe acute lower respiratory tract infections among patients younger than 2 years old. Arch Argent Pediatr 2017; 115 (3): 277–281. doi: 10.5546/aap.2017.eng.277.
4. Woodhead M, Blasi F, Ewig S et al. Guidelines for the management of adult lower respiratory tract infections – full version. Clin Microbiol Infect 2011; 17 (Suppl 6): E1–59. doi: 10.1111/j.1469-0691.2011.03672.x.
5. Ministry of Health, P. R. China. National guide to clinical laboratory procedures. 4th Ed. Beijing, China: People’s Medical Publishing House 2015.
6. Jean SS, Lu MC, Ho MW et al. Non-susceptibilities to antibiotics against important Gram-negative bacteria, and imipenem-relebactam, meropenem-vaborbactam against carbapenem non-susceptible Enterobacterales and Pseudomonas aeruginosa isolates implicated in complicated intra-abdominal and urinary tract infections in Taiwan, 2019. Int J Antimicrob Agents 2022; 59 (3): 106521. doi: 10.1016/j.ijantimicag.2022.106 521.
7. Li CH, Huang LN, Zhang MC et al. Forensic psychiatric assessment for organic personality disorders after cranio-cerebral trauma. Fa Yi Xue Za Zhi 2017; 33 (2): 158–161. doi: 10.3969/j.issn.1004-5619.2017.02.010.
8. Huang Q, Xu H, Xiao QS. Clinical research of different analgesia methods on perianesthetic pain of patients with moderate and severe craniocerebral injury who have emergency operation. Eur Rev Med Pharmacol Sci 2017; 21 (3 Suppl): 88–92.
9. Tonacio AC, Oliveira MS, Malbouisson LM et al. Trauma is associated with a better prognosis in intensive care patients with Acinetobacter infections. Infection 2014; 42 (1): 89–95. doi: 10.1007/s15010-013-0523-y.
10. Shengchen D, Gu X, Fan G et al. Evaluation of a molecular point-of-care testing for viral and atypical pathogens on intravenous antibiotic duration in hospitalized adults with lower respiratory tract infection: a randomized clinical trial. Clin Microbiol Infect 2019; 25 (11): 1415–1421. doi: 10.1016/j.cmi.2019.06.012.
11. Nguenha N, Tivane A, Pale M et al. Clinical and epidemiological characterization of influenza virus infections in children with severe acute respiratory infection in Maputo, Mozambique: results from the implementation of sentinel surveillance, 2014–2016. PLoS One 2018; 13 (3): e0194138. doi: 10.1371/journal.pone.0194 138.
12. Fitzner J, Qasmieh S, Mounts AW et al. Revision of clinical case definitions: influenza-like illness and severe acute respiratory infection. Bull World Health Organ 2018; 96 (2): 122–128. doi: 10.2471/BLT.17.194 514.
13. Zhong B, Sun SL, Du JT et al. Risk factors for lower respiratory tract infection in children with tracheobronchial foreign body aspiration. Medicine (Baltimore) 2019; 98 (10): e14655. doi: 10.1097/MD.0000000000014 655.
14. Cao C, Zhang L. Nursing care of lower respiratory tract infection after abdominal surgery. Cell Biochem Biophys 2015; 72 (2): 495–496. doi: 10.1007/s12013-014-0493-4.
15. Maccioni L, Weber S, Elgizouli M et al. Obesity and risk of respiratory tract infections: results of an infection-diary based cohort study. BMC Public Health 2018; 18 (1): 271. doi: 10.1186/s12889-018-5172-8.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2022 Issue 5
-
All articles in this issue
- Lumbar spine disorder – the new occupational disease
- Carotid web
- Development of the electronic memory test for the elderly (ALBAV)
- Outcomes of facial nerve reconstructive surgery
- Supracerebellar transtentorial approach
- The dentate gyrus – anatomy, vascular supply, function and neuropathology
- Computer-modeled cranioplasty from porous polyethylene in high-risk terrain
- Cenobamate
- Zemřel MUDr. Jan Pařízek
- Flora characteristics and risk factors of lower respiratory tract infections in patients with severe craniocerebral trauma in the intensive care unit
- Effect of sumatriptan following simulated traumatic brain injury in rats
- Bickerstaff brainstem encephalitis and Guillain-Barré syndrome overlap
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Lumbar spine disorder – the new occupational disease
- Cenobamate
- Outcomes of facial nerve reconstructive surgery
- Carotid web
