Spinální schwannom v oblasti hrudní páteře s masivním intratumorálním krvácením
Published in:
Cesk Slov Neurol N 2018; 81(5): 596-598
Category:
Letter to Editor
doi:
https://doi.org/10.14735/amcsnn2018596
Dear Editor,
Schwannomas (neurinomas, neurilemmomas) are benign, slow-growing tumours, arising from the Schwann cells of the nerve sheath. Spinal schwannomas have an incidence of 0.3–0.4 cases in every 100,000 persons per year and account for approximately 13% of all spinal tumours [1].
The classic pattern of growth of spinal schwannomas is dumbbell-shaped, with one part of the tumour occupying the spinal canal, extending in the intravertebral foramen towards the other part of the tumour, situated extraforaminally. The majority are subdural extrame-dullary, but completely extradural schwannomas can also be found. Asazuma has provided a comprehensive classification of spinal schwannomas based on the pattern of growth [2]. According to the histopathological characteristics, there are two types of tumours: Antoni A – firm tissue and Antoni B – loose tissue.
Patients usually present with radiculopathy followed by slowly progressive spinal cord compression syndrome.
The treatment of choice is always surgical, total resection being curative. Surgical resection of thoracic schwannomas could be performed using posterior, posterolateral, lateral, anterior or combined approaches.
Up to this point, there have been only a few cases of schwannomas presenting with intratumoural haemorrhage reported in the literature. We present a case of a thoracospinal schwannoma with acute intralesional bleeding.
A 72-year-old man was admitted to the neurosurgical department with abrupt onset of paraplegia. The patient had a history of high frequency atrial fibrillation and was undergoing treatment with oral anticoagulants, grade III arterial hypertension and grade II obesity. Two days prior to admission to our department, the patient presented thoracic pain and dyspnoea, followed by complete motor deficit in the lower limbs with sudden onset. The initial examination showed an international normalized ratio (INR) of 9.4. The cardiology exam excluded myocardial infarction or any other acute cardiovascular disorder. Doppler ultrasonography of the lower limbs revealed no signs of thrombosis. The cerebral CT scan excluded ischaemic or haemorrhagic stroke or subarachnoid haemorrhage (SAH). The patient was transferred to the neurosurgical department.
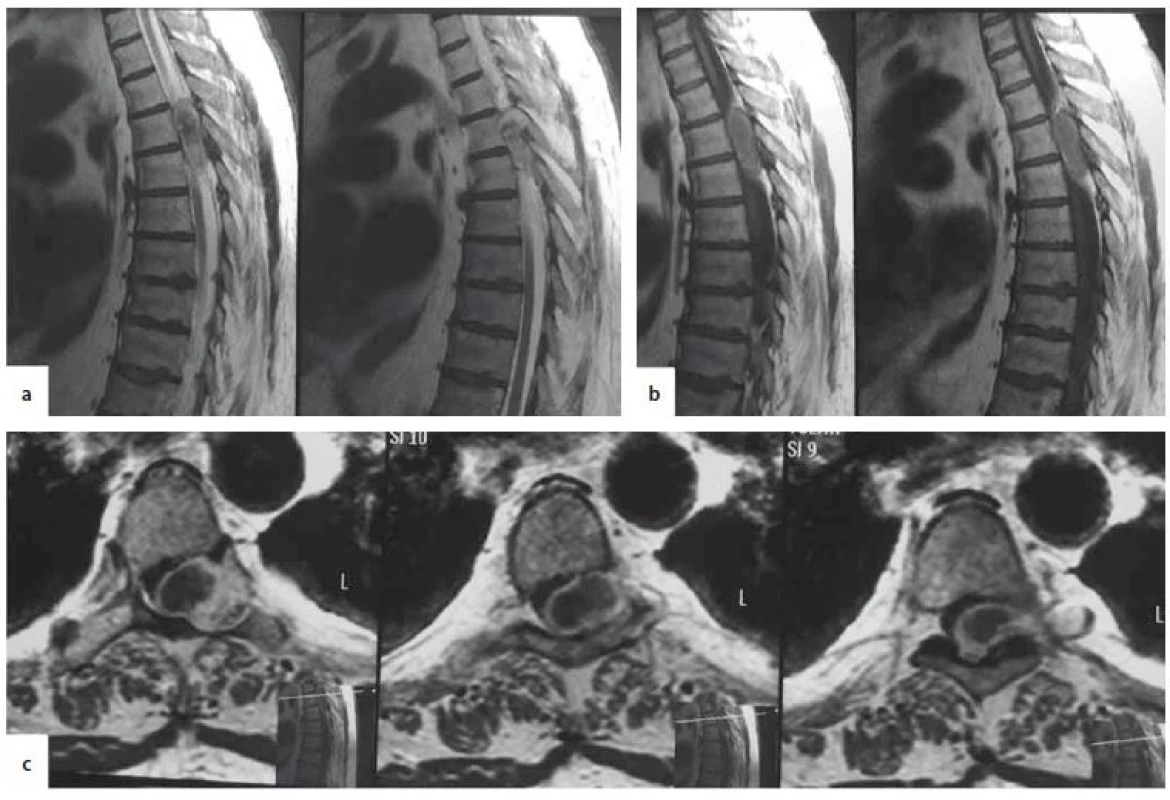
Upon admission, a neurological exam showed T6 American Spinal Injury Association score (ASIA). A paraplegia, absent deep tendon reflexes in the lower limbs, plantar reflexes, and cutaneous abdominal reflexes and loss of sphincter control. A spinal MRI revealed an extramedullary T5–T6 thoracic lesion. The lesion was inhomogeneous and isointense in T1 and T2 weighted images, with peripheral contrast enhancement. It occupied more than 2/3 of the spinal canal, compressing the spinal cord and had a dumbbell shape extending extraforaminally through the T5–T6 vertebral foramina. The lesion caused enlargement of the T5–T6 foramen on the left side and compression atrophy of the posterior arch of the T5 vertebra (Fig. 1).
T1W – T1-weighted image; T2W – T2-weighted image
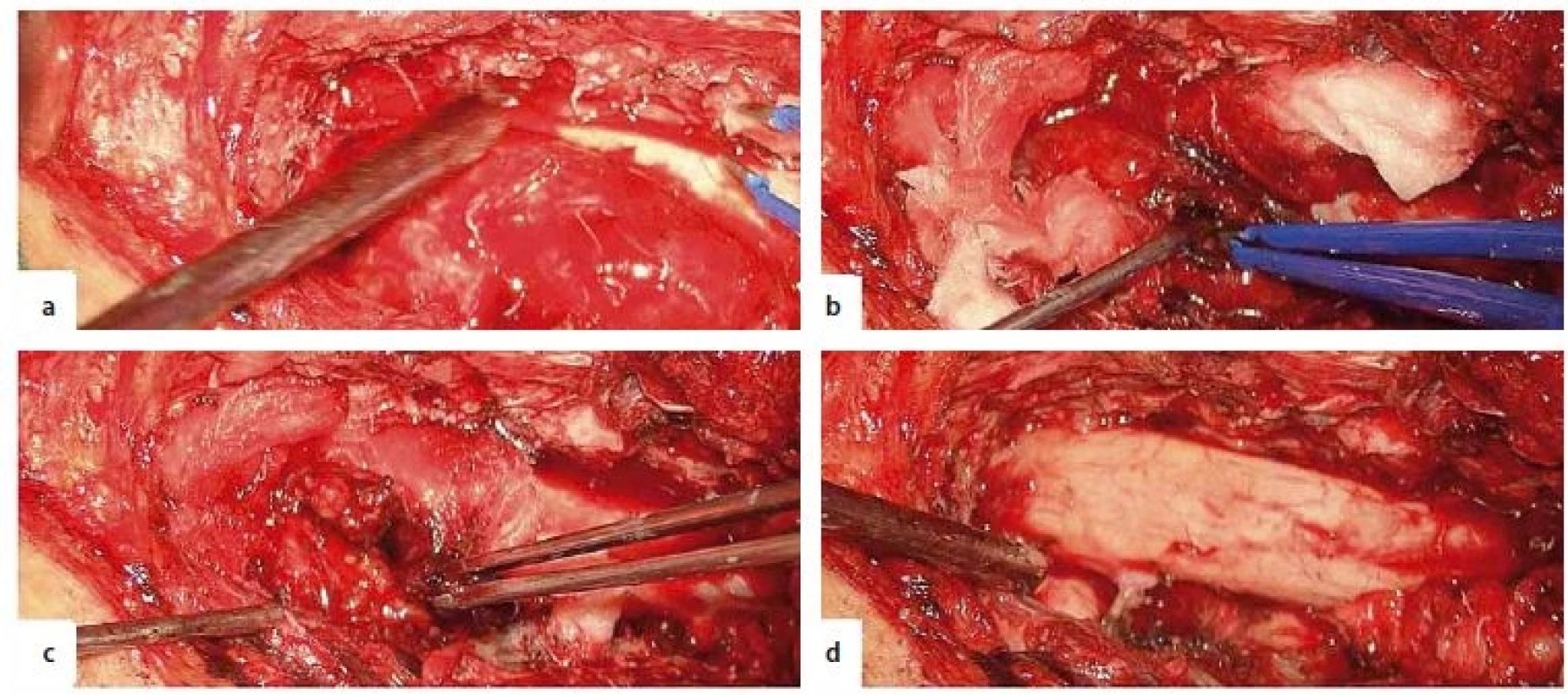
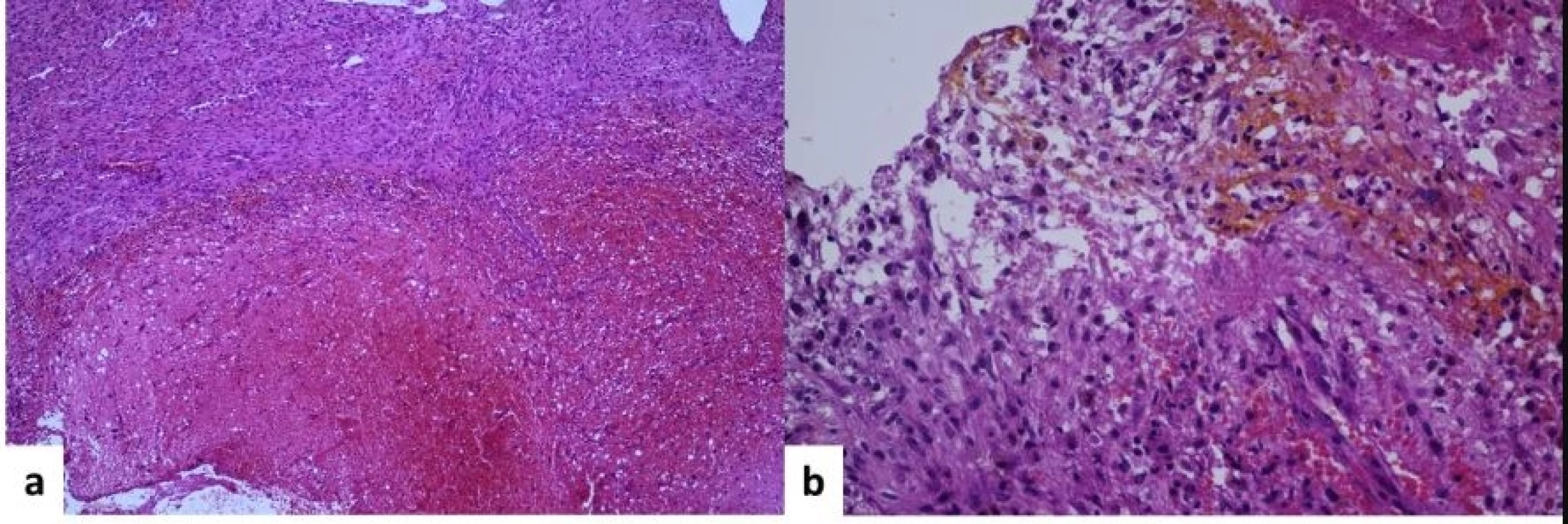
The patient underwent emergency surgery under general anaesthesia. He was positioned prone and the T5 vertebra was identified using a C-arm. Motor and somatosensitive evoked potentials in the lower limbs were recorded. We performed a longitudinal midline skin incision and exposed the posterior vertebral arches from T4 to T7. We performed decompressive T4–T7 laminectomies and left T5–T6 facetectomy. We found an encapsulated, red extradural tumour, Asazuma IIb, measuring 4.5 × 2.5 × 1.5 cm, causing massive compression of the spinal cord. The tumour was attached to the T5 left nerve root. A fenestration was made within the tumour capsule in order to perform intracapsular debulking. We found an intratumoural haematoma, which was evacuated. The extraforaminal part of the tumour was gradually brought into the surgical field by staged intracapsular debulking and progressively pulling the capsule inside the spinal canal. As the tumour was easily mobilised through the enlarged foramen, we did not need to perform an extracanalar approach. Finally, the tumour capsula was dissected free from its attachment from the T5 nerve root, achieving total resection (Fig. 2). To prevent CSF leaks, synthetic collagen was used to seal the dura. An epidural external drain was left in place and the wound was closed in anatomical layers. The histopathological exam revealed an Antoni A schwannoma with intratumoural haemorrhage (Fig. 3).
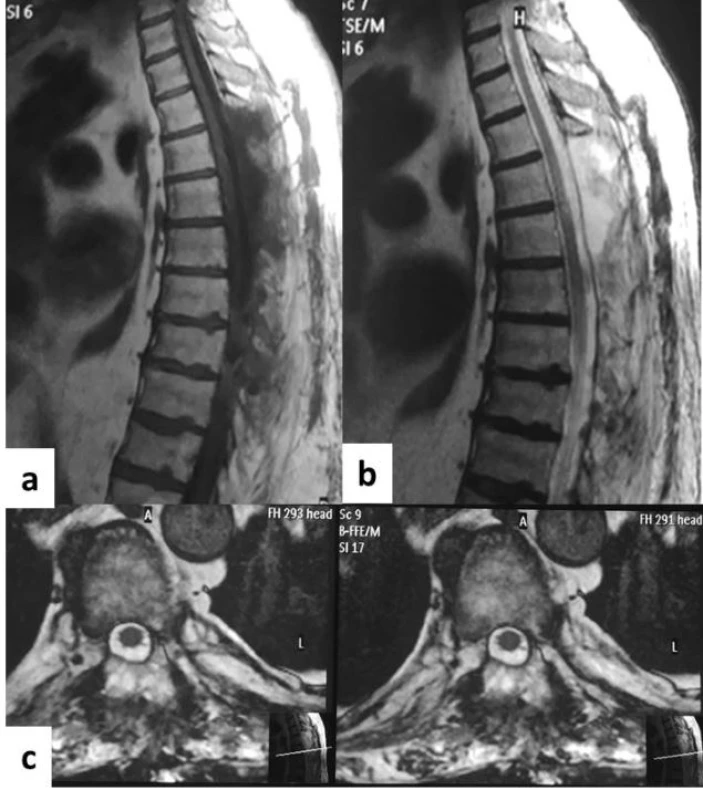
The patient presented no postoperative complications. In the 1st day following surgery, the patient regained sensitivity in his lower limbs and during the following days, he partially regained motor function in the right lower limb (ASIA 2). No sphincteral function was regained. Neurorehabilitation began on the 3rd day after surgery. A postoperative MRI showed T4–T7 laminectomies, complete resection of the tumour and reexpansion of the spinal cord (Fig. 4). The patient was transferred to a neurorehabilitation department.
Two months after surgery, he had full sensitivity and ASIA 3 motor function in his lower limbs and had regained sphincter control. At the 6-and 12-month follow-ups he had ASIA 4 motor function in his lower limbs.
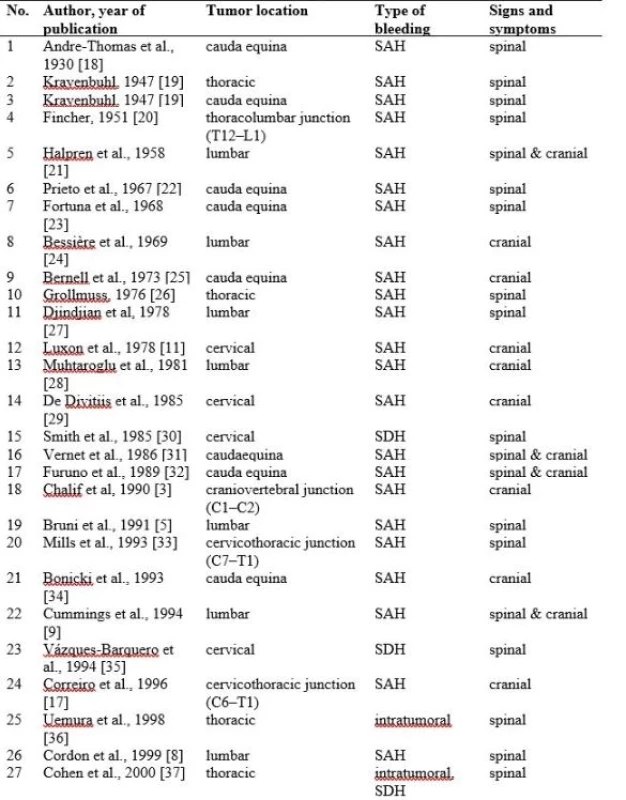

Schwannomas are slow-growing, benign tumours which only exceptionally present with haemorrhage. Because this form of presentation is very rare, such findings are presented only as case reports. Searching the relevant literature, we were able to find only 44 cases from 1930 to 2017. Spinal schwannomas with haemorrhagic onset can be found in any part of the spinal canal, from C1–C2 [3,4] to the cauda equina and the occurrence of bleeding is not influenced by the craniocaudal location. Patients with neurofibromatosis have no higher propensity to bleed from spinal schwannomas compared with sporadic schwannomas; one patient had multiple schwannomas [5] and one had neurofibromatosis type 2 [6].
Bleeding can occur intratumourally, in the subarachnoid space and/or in the subdural space. Intratumoural bleeding causes rapid tumour enlargement and patients present with sudden onset of spinal cord compression. Acute spinal cord compression syndrome can also be found in a spinal subdural haematoma. Depending on the quantity of the subarachnoid bleeding, due to the free circulation of fluids within this space, patients present intracranial SAH, either asymptomatic or with signs and symptoms of meningismus. SAH was the most common form of presentation, and that is why haemorrhagic spinal schwannomas should also be considered in the diagnosis of patients with SAH and negative cerebral angiography [7]. Intratumoural bleeding had been reported in only 12 patients until now.
There are a number of theories that can explain the possible mechanism of bleeding. The vascular theory [8] assumes that the tumour induces the formation of abnormal, hyalinized, ectatic vessels with turbulent blood flow, and thrombosis developing inside these vessels leads to consequent necrosis and bleeding. The presence of the tumour can induce local vascular changes such as stasis or arteriovenous shunts [9]. Torsion of the tumour or vascular pedicles causes vascular insufficiency with necrosis and bleeding [10]. The central ischaemic necrosis theory presumes that haemorrhage appears due to unequal balance between the tumour growth and neo-vessel formation. The latter mechanism can be encountered in malignant schwannomas or in schwannomas with a high rate of growth. The mechanical theory [11] implies rupture of the peritumoural vessels due to traction induced by tumour growth, or abnormal spinal movements, such as spinal manipulation procedures [12] or even delivery [13]. This latter theory explains bleeding from the schwannoma upper to the conus medullaris. Furthermore, secondary bleeding from the tumour may occur due to the misuse of oral anticoagulants [14,15] or traumatic events [16]. In the majority of cases schwannomas are poor vascular tumours. In rare cases schwannomas can be highly vascular [9], especially in the histopathological telangiectatic type [17]. Rarely, angiography can reveal a highly vascular mass related to a spinal schwannoma [9]. Our patient had long-term use of oral anticoagulants for a heart condition.
We consider that prolonged overdose of oral anticoagulants favoured bleeding from an already existing spinal schwannoma, because our patient had an INR upon admission of 9.4. The patient had no history of neurological deficits, although he had a benign spinal tumour, slowly growing for a long period of time, causing enlargement of the vertebral foramen and posterior arch atrophy compression. The patient became symptomatic only after acute bleeding occurred inside the tumour, causing massive and sudden expansion of the capsule. In our case this unusual onset was due to comorbidities.
Surgery for thoracic schwannomas con-sists in posterior (hemilaminectomy, laminectomy, ± facetectomy), posterolateral (transpedicular, costotransversectomy), lateral (lateral extracavity thoracic), anterior (transthoracic) or combined approaches. The surgery chosen for treatment of our lesion was a posterior approach T4–T7 laminectomy. Intracanalar extension of the tumour was from T5 to T6 in the craniocaudal direction. We chose this wide approach in order to get an access to the entire tumour mass and one level above and one level below the lesion. Besides good visualisation of both the tumour and levels above and below the lesion, a wide opening of the spinal canal is indicated in patients with acute spinal compression syndrome because it provides large decompression of the spinal cord. The thoracic spine is a very sensitive area, needing wide decompression to avoid the occurrence of vasospasms on the artery of Adamkiewicz. Articular facet joints T5–T6 on the left side were resected in order to facilitate the tumour resection, the tumour having a dumbbell shape extending both inside the spinal canal and extraforaminally. The left facetectomy was needed in a posterior approach to gain access to the intraforaminal and extraforaminal parts of the tumour. The posterior approach provides the widest spinal decompression and it is suitable in large tumours because the larger the tumour, the bigger the surgical corridor. We were able to preserve the nerve root, although in this location the nerve root can be safely sectioned if it cannot be dissected free of the tumour.
We did not perform laminoplasty at the end of surgery, because our purpose, besides the tumour resection, was wide decompression of the spinal canal. Following the tumour resection we decided not to perform posterior fixation, because the facet joints on the right side were preserved and the thoracic vertebral column is an extremely stable segment, thanks to the rib cage, thus spinal axial stability was not impaired by unilateral removal of the vertebral joints. Furthermore the patient was 72 years old and had osteoporosis, making screw insertion without cementation ineffective. Emergency surgery is the treatment of choice in patients presenting acute spinal cord compression, in order to decompress the spinal cord and restore the neurological functions. In our case, we received the patient more than 48 hours following the onset of motor deficit and although the patient underwent emergency surgery, he was unable to regain full motor function. This may be the reason that the results were not spectacular, but we have to keep in mind that even in the given conditions the postoperative outcome was favourable, sensory function was restored and motor function improved from ASIA 0 preoperative to ASIA 2 immediately after surgery, and to ASIA 3 in the first 2 months following the surgery. Furthermore, the patient regained sphincter control within the first 2 months.
The particularity of this case is unusual presentation with massive intratumoural bleeding in a thoracic schwannoma. This rare case suggests that neurosurgeons should be aware of the possible rapid evolution and abrupt neurological deterioration of a patient with a spinal schwannoma, especially when the patient is following chronic anticoagulant therapy.
Spinal schwannomas presenting with haemorrhage are extremely rare, only 44 cases have been reported since 1930. There are a number of theories that can explain intratumoural haemorrhage; however, one must not exclude secondary reasonsreasons for this type of bleeding, such as improper dosing of oral anticoagulants. Furthermore, we emphasise the need to include intratumoural bleeding in a spinal schwannoma as a differential diagnosis in patients presenting with abrupt neurological deficit. Surgery is the treatment of choice in patients with acute spinal cord compression and intracanalar lesions, to ensure a good neurological outcome.
Sources
1. Guha D, Davidson B, Nadi M et al. Management of peripheral nerve sheath tumors: 17 years of experience at Toronto Western Hospital. J Neurosurg 2018; 128 (4): 1226–1234. doi: 10.3171/2017.1.JNS162292.
2. Asazuma T, Toyama Y, Mariuwa H et al. Surgical strategy for cervical dumbbell tumors based on a three-dimensional classification. Spine (Phila Pa) 2004; 29(1): E10–E14. doi: 10.1097/01.BRS.0000103662.13689.76.
3. Chalif DJ, Black K, Rosenstein D. Intradural spinal cord tumour presenting as a subarachnoid hemorrhage: magnetic resonance imaging diagnosis. Neurosurgery 1990; 27 (4): 631–634.
4. Ciappetta P, D’Urso PI, Colamaria A. Giant craniovertebral junction hemorrhagic schwannoma: case report. Neurosurgery 2008; 62 (5): E1166. doi: 10.1227/01.neu.0000325881.21118.a6.
5. Bruni P, Esposito S, Oddi G et al. Subarachnoid hemorrhage from multiple neurofibromas of the cauda equina: case report. Neurosurgery 1991; 28(6): 910–913. 6. Inoue T, Miyamoto K, Kushima Y et al. Spinal subarachnoid hematoma compressing the conus medullaris and associated with neurofibromatosis type 2. Spinal Cord 2003; 41(11): 649–652. doi: 10.1038/sj.sc.3101496.
7. Parmar H, Pang BC, Lim CC et al. Spinal schwannoma with acute subarachnoid hemorrhage: a diagnostic challenge. AJNR Am J Neuroradiol 2004; 25(5): 846–850.
8. Cordon T, Bekar A, Yaman O. Spinal subarachnoid hemorrhage attributable to schwannoma of the cauda equina. Surg Neurol 1999; 51 (4): 373–375.
9. Cummings TM, Johnson MH. Neurofibroma manifested by spinal subarachnoid hemorrhage. AJR Am J Roentgenol 1994; 162(4): 959–960.
10. Jenkins AL 3rd, Ahuja A, Oliff AH et al. Spinal schwannoma presenting due to torsion and hemorrhage: case report and review of literature. Spine J 2015; 15(8): e1–e4. doi: 10.1016/j.spinee.2015.04.046.
11. Luxon LM, Harrison MJ. Subarachnoid hemorrhage and papilledema due to a cervical neurilemmoma: case report. J Neurosurg 1978; 48 (6): 1015–1018. doi: 10.3171/jns.1978.48.6.1015.
12. Hdeib A, Goodwin R, Sciubba D et al. Hemmorhagic thoracic schwannoma presenting with intradural hematoma and acute paraplegia after spinal manipulation therapy. Int J Spine Surg 2016; 10 : 42. doi: 10.14444/3042.
13. Tanaka H, Kondo E, Kawato H et al. Spinal intradural hemorrhage due to a neurinoma in an early puerperal woman. Clin Neurol Neurosurg 2002; 104(4): 303–305.
14. Motomochi M, Makita Y, Nabeshima S et al. Spinal subarachnoid hemorrhage due to a thoracic neurinoma during anticoagulation therapy – a case report. Neurol Med Chir (Tokyo) 1981; 21 (7): 781–784.
15. Ichinose T, Takami T, Yamamoto N et al. Intratumoral hemorrhage of spinal schwannoma of the cauda equina manifesting as acute paraparesis – case report. Neurol Med Chir (Tokyo) 2009; 49 (6): 255–257.
16. Nadeem M, Mansoor S, Assad S et al. Spinal schwannoma with intradural intramedullary hemorrhage. Cureus 2017; 9 (3) : e1086. doi: 10.7759/ cureus.1086.
17. Corriero G, Iacopino DG, Valentini S et al. Cervical neuroma presenting as a subarachnoid hemorrhage: case report. Neurosurgery 1996; 39(5): 1046–1049.
18. Andre-Thomas F, Schaeffer H, De Martel T. Syndrome d’hemorrhagie meningee realise par unetumeur de la queue de cheval. Paris Med 1930; 77 : 292–296.
19. Krayenbuhl H. Spontane spinale Subarachnoidalblutung und acute Rückenmarkskompression bei intraduralem, spinalem Neurinom. Schweiz Med Wochenschr1947; 77(25–26): 692–694.
20. Fincher EF. Spontaneous subarachnoid hemorrhage in intradural tumours of the lumbar sac. J Neurosurg 1951; 8(6): 576–584. doi: 10.3171/jns.1951.8.6.0576.
21. Halpren J, Feldman S, Peyser E. Subarachnoid hemorrhage with papilledema due to spinal neurofibroma. Arch Neurol Psychiatr 1958; 79(2): 138–141.
22. Prieto A, Cantu RC. Spinal subarachnoid hemorrhage associated with neurofibroma of cauda equina. J Neurosurg 1967; 27(1): 63–69. doi: 10.3171/jns.1967.27.1.0063.
23. Fortuna A, La Torre E. Neurinoma della cauda con emorragia subarcnoidea circoscritta. Il Lavaro Neuropsichiatrico 1968; 43 : 1157–1156.
24. Bessière E, Pouyanne H, Verin P et al. Hemorrhagic papillary stasis revealing a giant hemorrhagic neurinoma of the terminal cone. Neurochirurgie 1969; 15(3): 224–228.
25. Bernell WR, Kepes JJ, Clough CA. Subarachnoid hemorrhage from malignant schwannoma of the cauda equina. Tex Med 1973; 69(10): 101–104.
26. Grollmuss J. Spinal subarachnoid hemorrhage with schwannoma. Acta Neurochir (Wien) 1975; 31(3–4): 253–256.
27. Djindjian M, Djindjian R, Huth M et al. Spinal meningeal hemorrhage due to tumors: a report on 5 cases with arteriography (author's transl). Rev Neurol (Paris) 1978; 134(11): 685–682.
28. Muhtaroglu U, Strenge H. Recurrent subarachnoid haemorrhage with spinal neurinoma. Neurochirurgia (Stuttg) 1980; 23(4): 151–155. doi: 10.1055/s-2008-1053877.
29. De Divittis E, Maiuri F, Corriero G et al. Subarachnoid hemorrhage due to spinal neurinoma. Surg Neurol 1985; 24(2): 187–190.
30. Smith RA. Spinal subdural hematoma, neurilemmoma, and acute transverse myelopathy. Surg Neurol 1985; 23(4): 367–370.
31. Vernet O, Alaïli R, de Tribolet N. Spinal subarachnoid hemorrhage. Apropos of 2 cases of cauda equina tumors. Schweiz Med Wochenschr 1986; 116(23): 781–785.
32. Furuno M, Nishiura I, Koyama T. Spinal subarachnoid hemorrhage due to neurinoma of the cauda equina. No Shinkei Geka1989; 17(5): 485–488.
33. Mills B, Marks PV, Nixon JM. Spinal subarachnoid haemorrhage from an “ancient” schwannoma of the cervical spine. Br J Neurosurg 1993; 7(5): 557–559.
34. Bonicki W, Koszewski W, Marchel A et al. Caudal tumors as a rare cause of subarachnoid hemorrhage. Neurol Neurochir Pol 1993; 27(4): 599–603.
35. Vázquez-Barquero A, Pascual J, Quintana F et al. Cervical schwannoma presenting as a spinal subdural haematoma. Br J Neurosurg 1994; 8(6): 739–741.
36. Uemura K, Matsumara A, Kobayashi E et al. CT and MR presentation of acute hemorrhage in a spinal schwannoma. Surg Neurol 1998; 50(3): 219–220.
37. Cohen ZR, Knoller N, Hadani M et al. Traumatic intratumoral hemorrhage as the presenting symptom of a spinal neurinoma. Case report. J Neurosurg 2000; 93 (2 Suppl): 327–329.
38. Ng PY. Schwannoma of the cervical spine presenting with acute haemorrhage. J Clin Neurosci 2001; 8(3): 277–278.
39. Sun L, Chen Z, Jina F et al. Spinal schwannoma: an unusual cause of acute subarachnoid hemorrhage. Neurol India 2010; 58(1): 155–156. doi: 10.4103/0028-3886.60430.
40. Yeh HM, Leung JH, Huang KC et al. A long segmental hemorrhagic spinal schwannoma with atypical presentation. J Radiol Sci 2011; 36 : 191–196.
41. Kukreja S, Ambekar S, Sharma M et al. Cauda equina schwannoma presenting with intratumoral hemorrhage and intracranial subarachnoid hemorrhage. J Neurosurg Spine 2014; 21(3): 357–360. doi: 10.3171/2014.5.SPINE131014.
42. Sahoo RK, Das PB, Sarangi GS et al. Acute hemorrhage within intradural extramedullary schwannoma in cervical spine presenting with quadriparesis. J Craniovertebr Junction Spine 2015; 6(2): 83–85. doi: 10.4103/0974-8237.156069.
43. Zhang HZ, Li Y, Han Y et al. Spontaneous acute hemorrhage of intraspinal canal cellular schwannoma with paraplegia: a case report. Br J Neurosurg 2015; 29(3): 425–427. doi: 10.3109/02688697.2014.987212.
44. Zhang J, Huang Y, Meng Y et al. Spinal schwannoma hemorrhage manifesting as acute paraplegia. Spine J 2016; 16(10): e667–e668.
45. Zhang HM, Zhang YX, Zhang Q et al. Subarachnoid hemorrhage due to spinal cord schwannoma presenting findings mimicking meningitis. J Stroke Cerebrovasc Dis 2016; 25: e123–e125.
46. Prasad GL, Kongwad LI, Valiathan MG. Spinal intradural schwannoma with acute intra tumoural haemorrhage: case report and review. J Clin Diagn Res 2016; 10(1): PD01–PD03. doi: 10.7860/JCDR/2016/16187.7015.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2018 Issue 5
-
All articles in this issue
- Anaesthesia and neuromuscular disorders
- The best approach in motorized Parkinson‘s disease therapy is INTRADUODENAL LEVODOPA
- The best approach in motorized Parkinson‘s disease therapy is APOMORPHINE INFUSION
- The best approach in motorized Parkinson‘s disease therapy is DEEP BRAIN STIMULATION
- Cervical vertigo – fiction or reality?
- Care for patients with dysphagia after acute stroke in the Czech republic
- Computational fluid dynamics of intracranial aneurysms and its potential contribution in clinical practice from a neurosurgeon’s perspective
- Review of diseases with restricted diffusion on magnetic resonance imaging of the brain
- New insights in the diagnosis and treatment of amyotrophic lateral sclerosis
- Gamma knife stereotactic radiosurgery in recurrent or residual glioblastoma multiforme – our experience in two neurosurgical units
- Validation of questionnaire for patients with myotonia – Czech version of Myotonia Behaviour Scale
- The first documented case of Japanese encephalitis imported to the Czech Republic
- Early complication after treatment of dissecting intracranial aneurysm in vertebrobasilar circulation with a flow-diverter
- Harvey Cushing as Nobel Prize nominee
- Muscular dystopia in the fallopian canal
- Acute and subacute silent cerebral infarction in patients before elective coronary intervention
- Relationship between epidemiology and subjective perception of pain in patients with carpal tunnel syndome
- Speech intelligibility and clinical parameters in patients with Parkinson‘s disease
- The relationship of the basilar artery bifurcation and dorsum sel lae
- Spinální schwannom v oblasti hrudní páteře s masivním intratumorálním krvácením
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- New insights in the diagnosis and treatment of amyotrophic lateral sclerosis
- Review of diseases with restricted diffusion on magnetic resonance imaging of the brain
- Cervical vertigo – fiction or reality?
- Anaesthesia and neuromuscular disorders
