Intensive computer-assisted cognitive rehabilitation in persons with multiple sclerosis – results of a 12-week randomized study
Intenzivní rehabilitace kognitivních funkcí u osob s roztroušenou sklerózou – výsledky 12týdenní randomizované studie hodnocené počítačovým programem
Cíl: Účelem této studie bylo zhodnotit neuropsychologickou rehabilitaci pomocí 12týdenního počítačového programu, aby se zjistilo, zda to mělo vliv na zlepšení kognitivních funkcí; také k identifikaci metod, které lze použít k měření tohoto účinku. Cílem této studie je demonstrovat účinek zvoleného vzdělávacího plánu a výsledný stav kognitivních funkcí.
Metodika: Pacienti s diagnózou RS (43) byli randomizováni do dvou skupin – experimentální skupiny (26) a kontrolní skupiny (17). Všichni pacienti měli kognitivní defekt, který byl vyhodnocen na začátku studie. Po účasti na vzdělávacím programu byly výsledky monitorovány pomocí neuropsychologických testů. Účastníkům experimentální skupiny byla poskytnuta rehabilitace kognitivních funkcí pomocí počítačového tréninkového programu, který absolvovali doma. Proběhlo 32 školení, která se konala ve stanovených dnech s konkrétním podrobným plánem školení.
Výsledky: Neuropsychologické testy použité na začátku a na konci studie prokázaly pozitivní účinek vzdělávacího programu. K největšímu zlepšení došlo v oblastech krátkodobé paměti a pozornosti.
Závěr: Výsledky ukázaly, že u těch pacientů s RS, kteří se řídili plánem počítačového výcviku, došlo k pozitivním účinkům neuropsychologické rehabilitace.
Klíčová slova:
roztroušená skleróza – neuropsychologická rehabilitace – pozornost – paměť – počítačový kognitivní trénink
Authors:
D. Chmelařová 1,2; L. Fiala- 1 3; M. Dostál 4; J. Lenz 5,6
Authors place of work:
Department of Psychiatry, First Faculty of Medicine, Charles University, Prague, Czech Republic
1; Department of Psychiatry, Faculty of Medicine, Charles University, Pilsen, Czech Republic
2; Institute of Sexology, First Faculty of Medicine, Charles University, Prague, Czech Republic
3; Department of Computer Science and Engineering, University of West Bohemia, Czech Republic
4; Department of Pathology, Znojmo Hospital, Czech Republic
5; Department of Anatomy, Histology and Embryology, Faculty of Veterinary Medicine, University of Veterinary and Pharmaceutical Sciences Brno, Czech Republic
6
Published in the journal:
Cesk Slov Neurol N 2020; 83/116(4): 408-415
Category:
Původní práce
doi:
https://doi.org/10.14735/amcsnn2020408
Summary
Aim: The purpose of this study was to evaluate neuropsychological rehabilitation using a 12-week computer program to assess if it had an effect on improving cognitive functions and to identify methods that can be used to measure this effect. The aim of this study is to demonstrate the effect of the chosen educational plan and the resulting state of cognitive functions.
Methods: Patients diagnosed with MS (43) were randomized into two groups – the experimental group (26) and the control group (17). All patients had a cognitive defect that was assessed at the beginning of the study. After participating in the training program, the results were monitored using neuropsychological tests. Participants in the experimental group were given their rehabilitation of cognitive functions using a computer training program which they undertook at home. There were 32 training sessions which took place on predetermined days with a specific detailed training plan.
Results: The neuropsychological tests used at the beginning and the end of the study showed the positive effect of the training program. The greatest improvement was seen in the areas of immediate memory and attention.
Conclusion: The results showed that in MS patients who followed the computer training plan, there were positive effects of the neuropsychological rehabilitation.
Keywords:
memory – multiple slerosis – neuropsychological rehabilitation – attention – computer cognitive training
Introduction
Multiple sclerosis is one of the most common neurological diseases and, at the same time, one of the most common causes of chronic neurological disability in young adults. MS leads to physical disability as well as cognitive and neuropsychiatric symptoms. Cognitive disorders and physical disability can occur independently. This in turn can complicate recognition or assessment of the disease. Cognitive deficiencies are most often characterized by mild cognitive impairment and, at the more advanced stages, by subcortical dementia. The first studies have already confirmed that the deficit is most commonly observed in complex attention, efficiency of information processing, processing speed (information), executive functions and long-term memory [1]. During the illness, cognitive changes can occur at any time and sometimes as the primary symptom. Generally, any two patients with this disease do not have exactly the same symptom profile or disease progression [2]. Cognitive disorders are hardly noticeable at first, which is the reason why they have escaped the attention of physicians for years. Previous neurological studies have identified that cognitive function deteriorates in 50–75% of patients [3]. Over the last 10 years, the diagnostic criteria and medications have improved to modify the disease, which in turn leads to earlier diagnosis and treatment. Furthermore, good cognitive training can minimize the impact of the illness on the patient’s quality of life. However, at the moment, the methods of such training are not unequivocally methodically or therapeutically fixed. For this reason, studies over the last decade have been trying to address the question of whether there is an effective rehabilitation strategy which could lead to the circumnavigation of damaged brain structures and restoration of cognitive functions due to the plasticity of the brain and the ability to restructure neural networks [4]. Previously, the training was predominantly focused on learning and memory functions. However, attention is now being transferred to the training of executive functions and attention [5]. Co-incidentally, there have been studies confirming that restructuring of the neural networks within the CNS is possible under the influence and in response to external stimuli, injuries or environmental changes [6]. Nevertheless, there are only a few studies that have investigated the mechanisms of targeted rehabilitation in detail. Also, the studies that are available provide only fragmented or incomplete data [7]. In 2013, Mantynen et al published a randomized controlled trial that involved 102 patients with relapsing remitting MS. Those authors concluded that neuropsychological rehabilitation did not necessarily improve cognitive performance, but reduced the perception of the patient’s cognitive deficit and thereby positively influenced their quality of life. What that meant was that although the effect had not been clearly demonstrated, the patients were feeling better [8]. What the results of this study showed is that focusing on the emergence of randomized controlled trials with a sufficient number of patients is commendable. Nevertheless, it is clear that even the results of these studies may not be relevant to the assessment of the effect of the rehabilitation of cognitive functions. To be precise, these studies showed that accurately targeted training is necessary for a demonstrable positive effect (compared with any kind of training), and what we consider to be very important is the correct time distribution and sufficient frequency of repetition. In the years 2014–2015, two studies were published that provided a systematic overview of the experience of cognitive function training in patients with MS. The first was a study focused on the identified conflicting findings in the published literature about the effectiveness of various forms of cognitive rehabilitation techniques used in patients with MS [5]. The second was a meta-analysis assessing the effects of cognitive intervention in MS, including only randomized controlled trials with comparable conditions [9]. Both studies agreed that the training of cognitive functions had a positive effect on patients and that this training should be an integral part of the overall care for patients with MS. Nevertheless, these studies also pointed to the contradictory results of previous research. These contradictions were in terms of the training methods used, the training plans and measurement techniques. Some of the frequent methodological problems include a small sample size, absence of a control group and problems related to output measurements. This insufficient quality of output measurement may be the reason why we do not record all the possible changes and therefore do not capture the possible positive effects [5]. A systematic review published in 2015 compared old and new studies, described the current state of the field and suggested the direction for ongoing MS research. The authors concluded that training of patients in psychiatric activities resulted in the improvement in cognition and brain functions. However, it is not clear to what extent the brain is capable of plasticity. This research identified contradictory results related to the efficacy of various rehabilitation techniques. Therefore, no definitive conclusions can be drawn on the effect of cognition, mood, quality of life, fatigue or how patients perceive this effect. Another factor that may also influence the results is the selection of the output measurements that were used in these studies and may not be capable of ascertaining all the possible effects. If output measures are not able to detect the changes, it does not mean that the examined rehabilitation exercise is not effective; it rather means that an insufficiently sensitive output measure has been used instead, i.e., a possible positive effect has not been detected, which, in turn, has likely led to incorrect conclusions [5].
All of the above-mentioned facts have had an influence. The research team of the department of psychiatry has tried to create and implement a rehabilitation procedure without the known negative factors and find an evidence-based rehabilitation process. The authors have therefore attempted to create a recommended set of procedures related to the rehabilitation of cognitive function in patients with MS, which are based on the conclusions of the studies discussed in this and other articles, and, at the same time, tried to evaluate this procedure in the form of a randomized study with a control group.
Materials and methods
Patients
The following inclusion criteria were used: a diagnosis of MS, the score of 0–6 on the Expanded Disability Status Scale (EDSS), age 18–65, a functionally dominant upper limb (in order to operate a keyboard), and access to a computer with an internet connection in the training environment. The following patients were excluded from the study: patients with a history of drug or alcohol abuse, major psychiatric disorders, acute relapses, neurological diseases other than MS, or patients with an ongoing rehabilitation. Hospital records were examined in order to verify the information obtained from the patients regarding their past medical history.
Eighty patients were put forward to participate in this study. However, 22 patients did not meet the inclusion criteria. Fifty - -eight patients were randomized into the experimental and control groups. Thirty-five patients were assigned to the experimental group and six patients from this group were excluded due to the lack of training conditions. Other three ones were excluded for health reasons. Statistical analyses comprised 26 patients (22 females) in the experimental group. Twenty-three patients were randomized to the control group. From this control group, six patients were excluded for health reasons. Statistical analyses comprised 17 patients (12 females). The ‘health reasons’ for exclusion in both groups were: any health problems with a duration of more than 3 days or a change of medication during the study.
There were no significant differences in age (the experimental group had an average age of 41.3 years [SD = 6.5], the control group had an average age of 42.4 years [SD = 9.2], Mann-Whitney U test, U = 231, Z = 0.19 [Pearson Chi-square: 1.22, df = 1; P = 0.27], education [Pearson Chi-square: 1.30, df = 3; P = 0.73], level of disability (Pearson Chi-square: 2.3, df = 3; P = 0.51), or EDDS score (the experimental group had an average score of 3.1 [SD = 1.4], the whole control group had an average score of 3.3 [SD = 2.0]; U = 218.5, Z = –0.06; P = 0.95).
Interventions and outcome assessment
The selected and evaluated group was designed to monitor the functions targeted by the software, while, at the same time, it was sensitive enough to recognize any changes in patients with mild cognitive defects. The patients in the selected and evaluated group were subjected to carefully chosen testing techniques and self-assessment questionnaires that were selected according to the results from previous studies. These studies were where positive results were found only in the testing methods and not in the subjective evaluation [10] or purely in the subjective evaluation of the patients [11]. Furthermore, the authors also included techniques related to possible changes in the area of emotions.
The neuropsychological testing
- Repeatability of the results on the selected and evaluated group for the Assessment of Neuropsychological Status (RBANS), Christopher Randolph. The RBANS is a brief, individually administered test, which measures attention, language, visuospatial/constructional abilities and immediate and delayed memory. The test comprises 12 subtests that can be administered by trained examiners in about 20 to 30 min. RBANS is intended for the use in adolescents and adults, aged 12 – 89 years.
- Trail Making Test (TMT) – The Trail Making Test is a neuropsychological test of visual attention and task switching. It consists of two parts in which the subject is instructed to connect a set of 25 dots as quickly as possible while still maintaining accuracy. The test can provide information about visual search speed, scanning, speed of processing, mental flexibility, as well as executive functioning. It can sensitively detect cognitive impairment. This test is a part of the Halstead-Reitan Neuropsychological aspect of the selected and evaluated group.
Questionnaires and measures
The self-assessment questionnaires and measures:
- The Cognitive Failures Questionnaire (CFQ) [12] is a self-assessment questionnaire that focuses on the examination of cognitive function disorder in routine daily activities. A range of somatic well-being and a range of psychological well--being are self-assessment measures related to the current psychological and somatic well-being. These are 11-point Likert type scales ranging from 0–10.
- The Schwartz SOS 10 scale is a 10-item scale intended for the field of psychiatry and psychology with regard to the evaluation of therapy. The purpose of this measure is to assess the effectiveness of treatment across a wide range of treatment facilities and various populations. It has been shown to have good psychometric properties [13].
- Beck Depression Inventory II (BDI-II) is a psychodiagnostic questionnaire assessing the presence and severity of depression. This questionnaire is frequently used in the field of clinical psychology and neuropsychology as a screening measure of the actual severity of depression.
The objective measure:
- Hamilton‘s Scale of Depression (HAMD) [14].
All the test techniques have been demonstrated in the form of a pencil-paper.
The neuropsychological rehabilitation
All patients participated in an entry examination that consisted of a neuropsychological selection and evaluation of the group. Subsequently, patients in the experimental group received training on the Happy Neuron Brain Jogging computer program, which they were later asked to work on at home. Cognitive online training HAPPY neuron Brain Jogging was created by ABET HOLDING, a. s. (Liberec, Czech Republic), which is a part of the French company SBT Group (Prague, Czech Republic).
The program contains 20 different tasks related to memory, concentration, speech, logical thinking, special orientation, and other abilities. It also offers training. Different levels of difficulty can be set thereby achieving a high variability in the exercise. This program also includes “an automatic coach”, which is able to select the appropriate set of exercises to optimize the benefit for the patient. Therefore, this program can also be used by patients that are currently unable to train under the direction of a clinical psychologist or a neuropsychologist.
The training plan was designed to run 4 times per week / 30 min per session for 8 consecutive weeks. Overall, there were 32 training sessions on predetermined days with a specific training plan.
The primary goals of the program included the following cognitive functions:
- memory
- attention and concentration
- speed and information processing
- executive functions
- expression and speech comprehension
- spatial orientation and perception.
The training was primarily focused on the improvement of attention concentration, memory stimulation, improvement of logical thinking and the expansion of vocabulary. Another primary goal was to achieve improvements in the area of immediate memory, speech, delayed recognition and visual-spatial perception.
All patients were given a training sheet which included two exercises, which were the same for all participants, with the requirement of repeating the exercise three times. For the remaining time (the total time was 30 min), participants were asked to undertake an exercise of their choice. For example, if the participant completed the required exercise in 25 min, he / she could choose another exercise for the remaining 5 min. The patient also received a written training instruction that it is better to repeat one exercise multiple times rather than performing exercises only once. The patients were required to complete all 32 training blocks. The results for each training session were immediately recorded after the particular session on a designated website. If and when needed, communication with patients was also carried out through this website. All other communication was performed through email and phone calls. If the administrator noticed that the training had not been completed, the patient was contacted to discover the reason and to jointly work out how to continue the training.
The control group received no training. In order to control the placebo effect, the control group was repeatedly contacted for a period of 2 months (3 times in total) and asked to report their current psychological status by completing a prepared questionnaire. The recorded and quantified improvement of the control group was deducted from the results of the experimental group.
In the final phase of the trial, all patients from the experimental and control group completed a final neuropsychological testing.
The training program was precisely designed by a neuropsychologist and targeted to tracking domains. Patients enrolled in the study had no other forms of neurorehabilitation.
Results
Results of the neuropsychological batteries
The experimental group participated in a home computer rehabilitation program of cognitive functions over 8 consecutive weeks (4 times per week / 30 min per session). Overall, there were 32 training sessions that took place on predetermined days with a specific training plan. The control group received no training and kept only minimal contact with the therapeutic team. Patients in the control group were contacted 3 times and asked to complete a questionnaire about their current mental health state.
After 8 weeks of initial testing, both the experimental and control groups were screened by neuropsychological batteries with the following results (Tab. 1). Table 1 shows the total score of the initial (RBANS 1) battery and the follow-up (RBANS 2) scores – the average results for the entire group and the standard deviation.
Initial examination, prior to the training, showed that the experimental and control groups did not significantly differ in the total score or in any of the five given RBANS sub-groups. However, following the training, the experimental group received higher scores in all given variables and obtained a significantly higher total score (Mann - -Whitney U test, Z = 2.411; P < 0.05), and significantly higher scores on Immediate memory (U test, Z = 1.982; P < 0.05), Attention (Z = 2,444; P < 0.05), and Delayed recognition (Z = 2,277; P < 0.05).
The experimental group showed statistically significant improvements in the total RBANS score and in some of the RBANS sub-groups with the exception of Speech (Tab. 1). The whole control group showed statistically significant improvements for Short-term memory (P < 0.05). No other significant changes were observed in the control group.
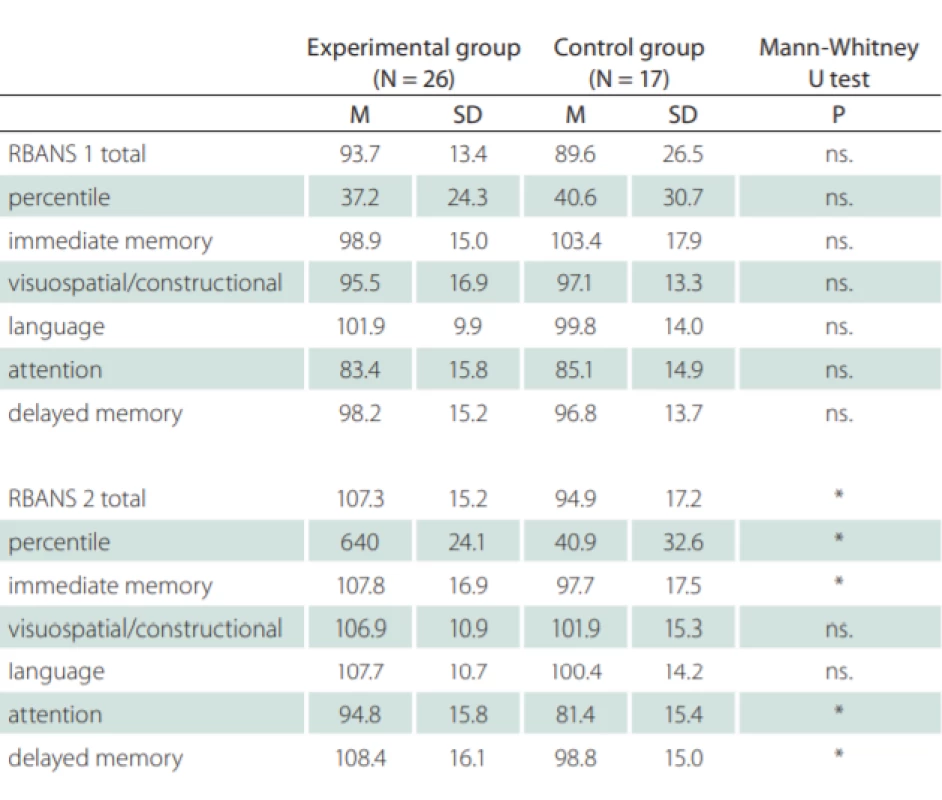
M – mean; N – number; ns. – non-signifi cant; RBANS – Repeatability of the results on the selected and evaluated group for the Assessment of Neuropsychological Status; SD – standard
deviation
The experimental group also improved in all RBANS variables with a minimum of 5.8 points (5.7% increase) in the Language domain, and up to 13.6 points (14.5% increase) in the total score. The control group even showed some deterioration in some of the variables, where the smallest change was –5.7 points (–5.5% drop) in the Immediate memory domain to a maximum improvement of 5.3 points (5.9% increase) in the total score. An essential part of the project was also the exclusion of “practice effect” from the results of the study. This exclusion was achieved by comparing the results of the control and experimental groups, where the work of the control and experimental groups was the same, except for the targeted cognitive training that was fulfilled only by the experimental group. If we consider the total score of the control group as a “practice effect” (with no intervention in the sense of targeted cognitive training) and deducted this effect (5.3 points), the experimental group would still show statistically significant net improvement of the intervention by 8.3 points (Tab. 2). The Mann-Whitney U test shows statistical probativeness of a given indicator – “ns.” for non-significant.
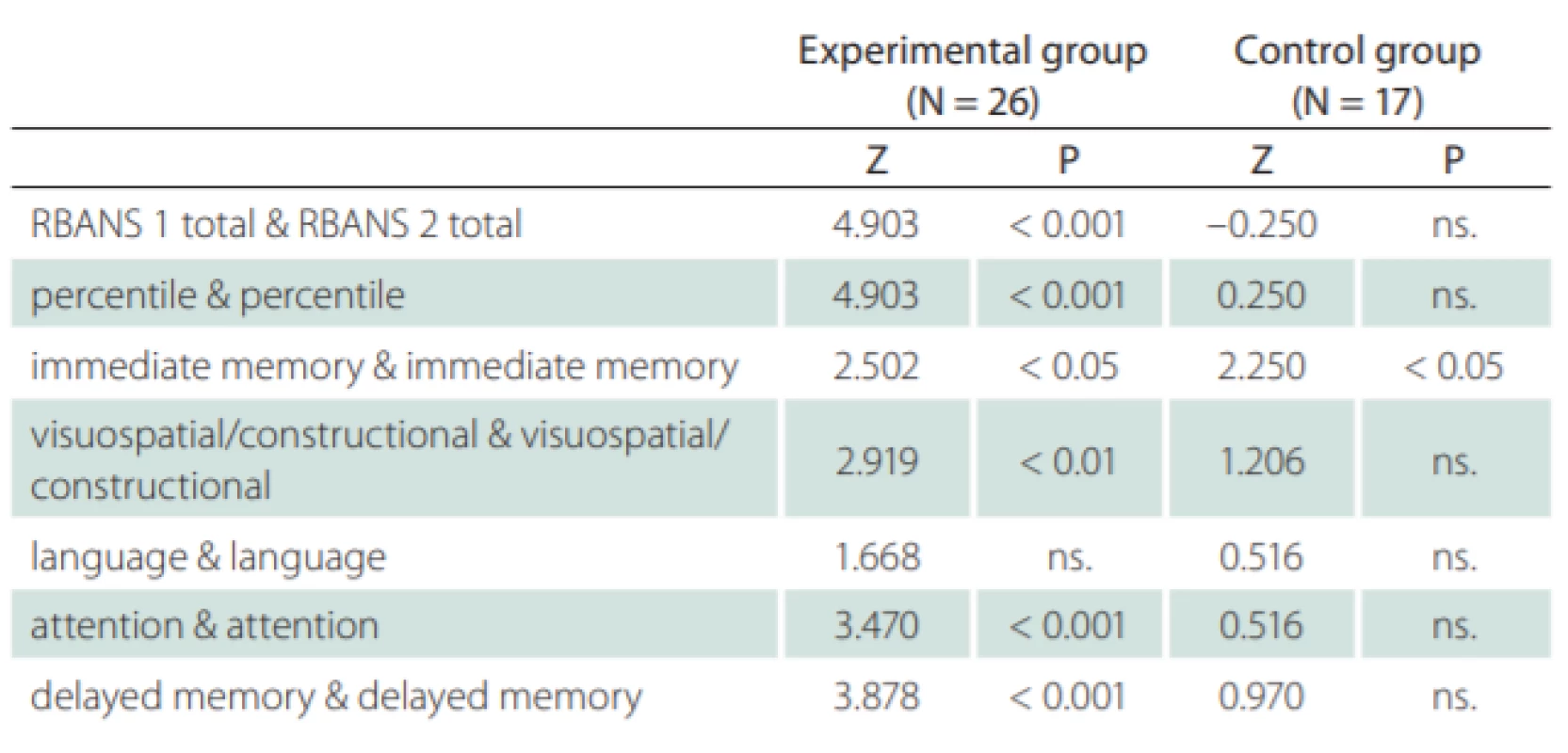
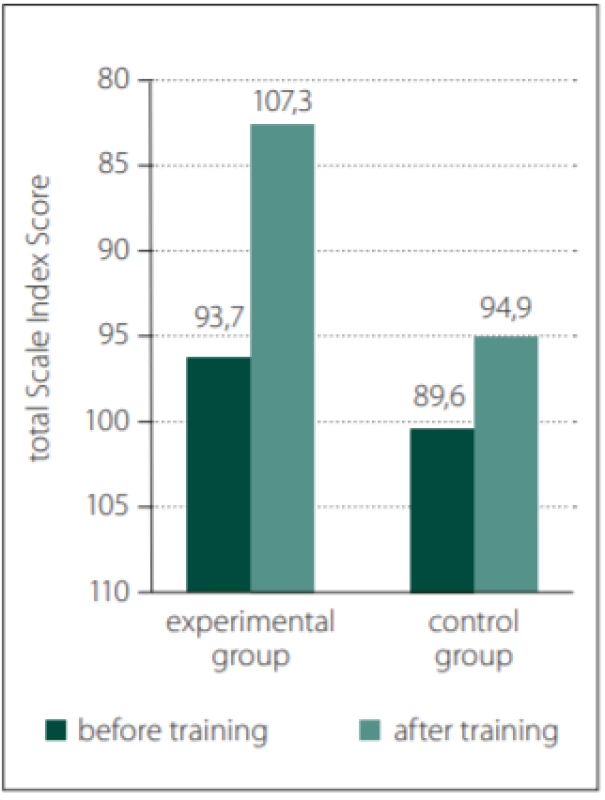
Table 2 clearly shows a statistically proven positive effect related to the cognitive function training. The overall results for the control and experimental groups are shown in Fig. 1. The figure clearly shows improvements for the experimental group.
Obr. 1. Celkové výsledky pro výzkumnou
a kontrolní skupinu.
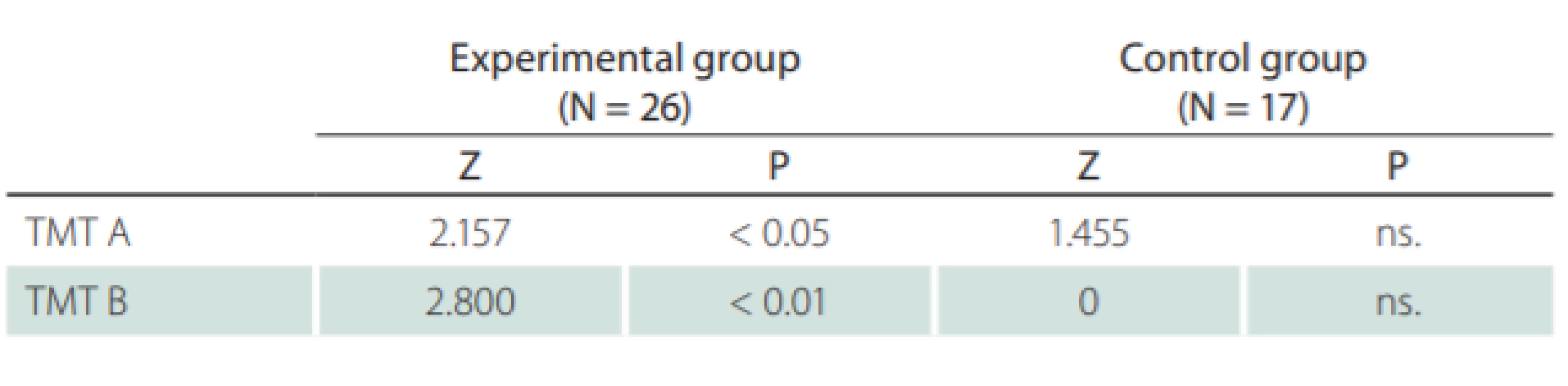
The TMT neuropsychological test was used in this study with the following results: the initial comparison of both groups showed no significant differences. The comparison of the results for both groups in terms of the entry and exit examinations is shown in Tab. 3. The TMT test results showed significant improvement in attention concentration, even after the “practice effect”. This was measured within the control group and taken into account.

Results of the self-report measures and other cognitive tests
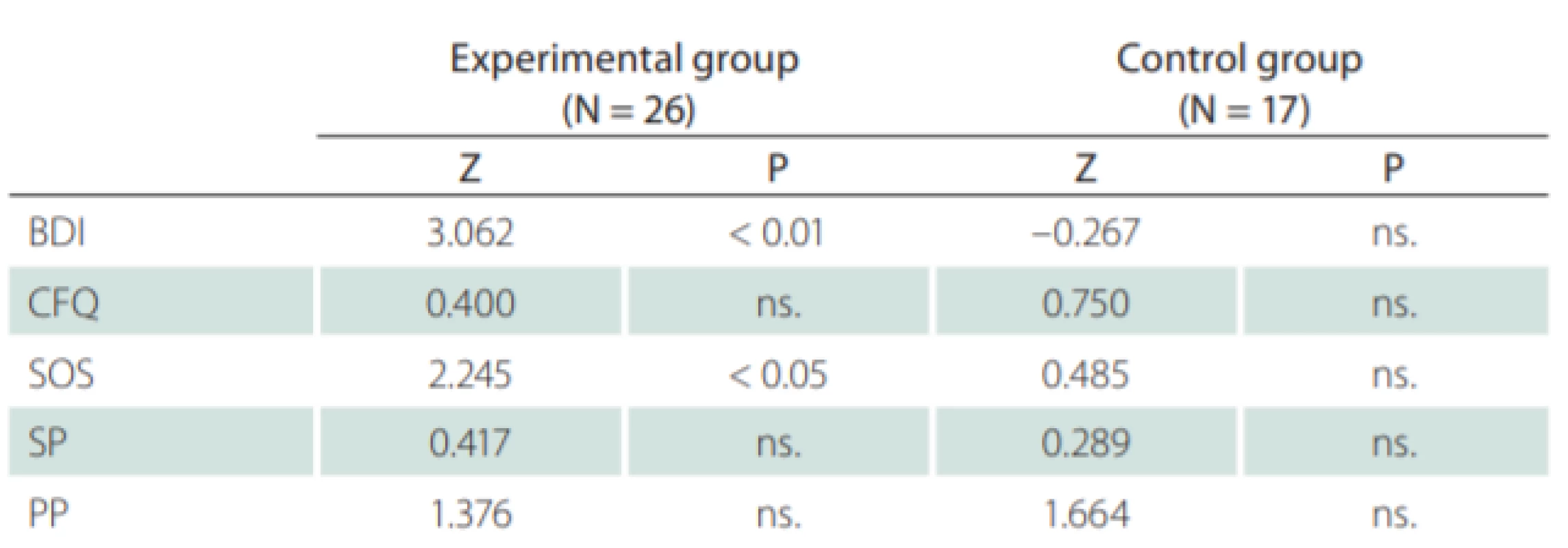
No statistically significant differences for the experimental and control groups were observed for all used methods prior to the training. Likewise, no statistically significant differences were recorded after the training. The evaluation of the results for these techniques is shown in Tab. 4.
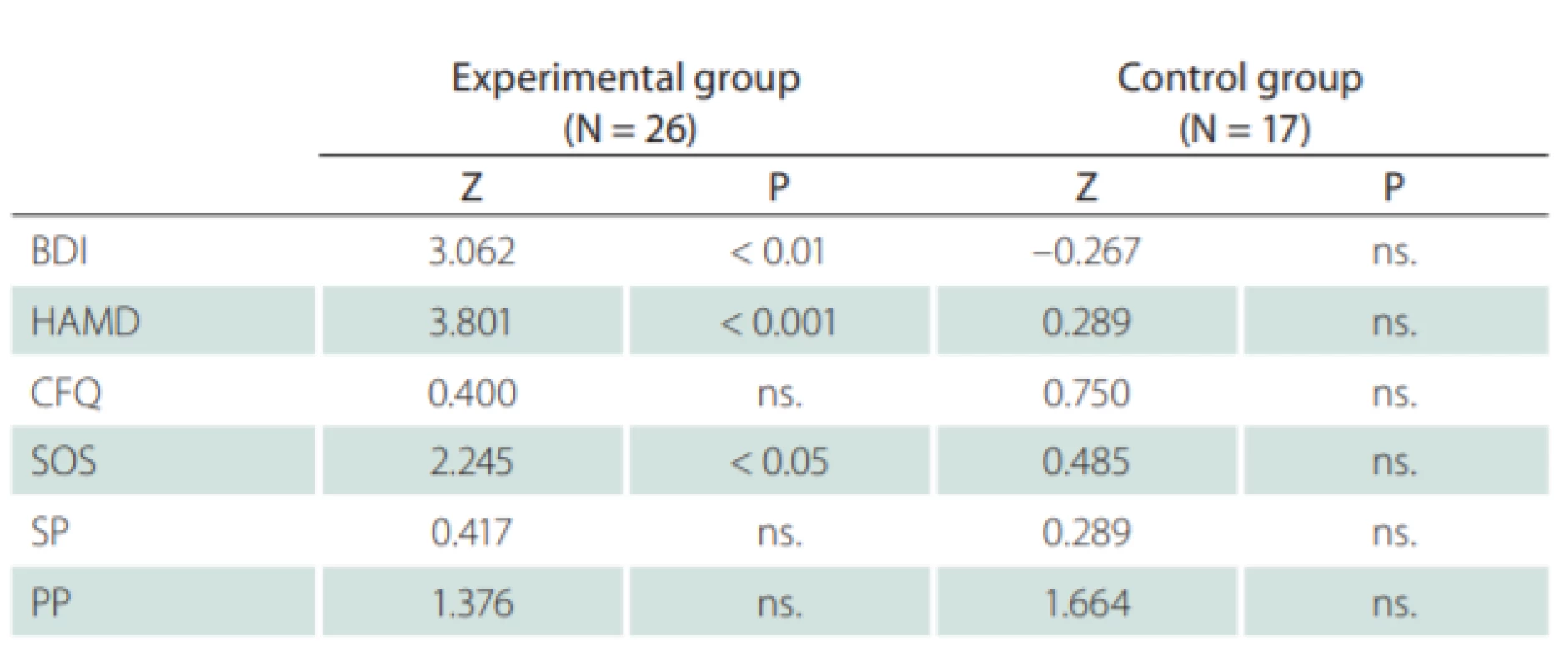
Comparing the data of the self-assessment measures gathered during the entry and exit interviews, the results were demonstrably better in favor of the experimental group. An improvement was seen across all five scales, while the improvement in the most objective BDI scale was statistically significant – on average 4.6 points. Nonetheless, some improvement was also seen in the control group and, when standard deviation was included in the comparison, no statistically significant improvement was observed in the experimental group. Thus, the improvement in subjective perception can occur without targeted cognitive training, particularly if the patient feels that enough time has been devoted to him / her. As for the objective indicators, however, this improvement was not observed.
The overall evaluation
In terms of assessing the results, subjective and objective techniques and measures have to be evaluated separately. Statistically significant improvements in the experimental group were observed in nearly all the objective tests (Tab. 5). As for the self-assessment scales, the results were less pronounced (Tab. 6).
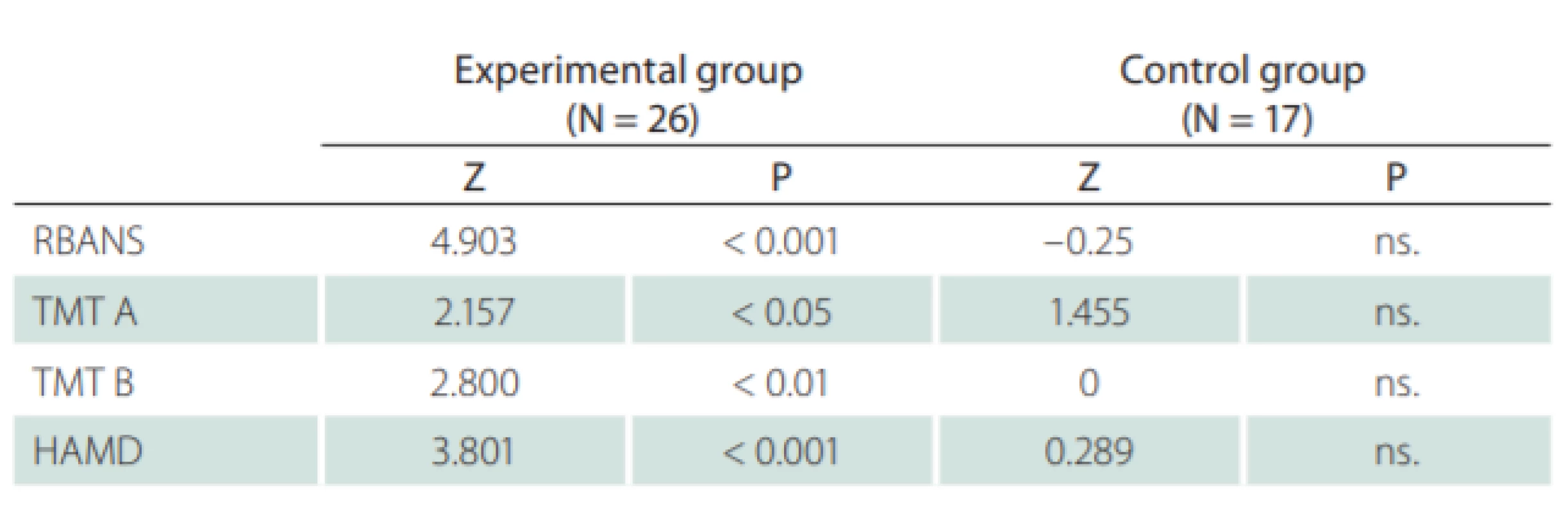
When comparing the objective with the self-assessment measures, it was clearly shown that the objective techniques demonstrated a significant positive effect relating to the cognitive training conducted within this study. By using only the self-assessment measures (subjective), the positive effect of such training would not be recognized.
As for the most efficient RBANS test in the selected and evaluated group, a significant improvement was observed in the immediate memory and in the Visuo-spatial/Constructional sub-scores. On the contrary, the lowest score was found in the Language sub-score. The results of this study do not clearly explain the difference in the effect of the cognitive training preformed for the various categories of cognitive functions. We assume that a significant influence lies within the fact that the proposed cognitive exercises almost always contain a memory component, i.e., even exercises related to speech function train this memory component while also training attention.
Discussion
The focus of many studies was the effectiveness of cognitive training. The early studies focused on the training of cognitive functions in patients with traumatic brain injury [15]. Relatively few studies were conducted on the treatment of cognitive disorders. Some studies revealed the benefits of cognitive rehabilitation for people with MS while others showed no improvement. The conclusions of these studies were, however, limited by methodological problems, such as initial differences between groups, the use of qualitative rather than quantitative research, and the need to rely on case studies. This prompted the need for a methodologically thorough research, ideally with a placebo-controlled, randomized design [2]. In 2013, Mantynen et al published a randomized controlled trial involving 102 patients with relapsing remitting MS. Patients were randomly assigned into two groups, a training group and a control group. The training group received rehabilitation for cognitive functions once a week in 60-min sessions for 13 weeks. The control group received no training. Neurological entry and exit interviews were conducted in both groups. The authors concluded that neuropsychological rehabilitation did not improve cognitive performance, but reduced the perception of patients’ cognitive deficits and thereby positively influenced their quality of life. This means that although the effect was not clearly demonstrated in the testing material used, patients reported as feeling better, subjectively [8]. Other studies showed the importance of taking into account that plasticity represents the basic developmental ability of the brain, learning, and memory even in healthy individuals. In the context of MS, this term (plasticity) encompasses molecular, synaptic, and cellular events, and even the reorganization of the cortex or fibers that are necessary to recover from acute or chronic damage to recovery. Very promising methods for the evaluation of this theory are MRI, functional MRI (fMRI), and DTI imaging [16]. The use of these advanced MRI techniques in MS patients recently demonstrated that the plasticity and functionally relevant long-term reorganization processes were preserved in the most advanced stages of the disease, and that these phenomena were functionally important to maintain motor and cognitive functions. Nonetheless, only a few studies explored the mechanisms of the targeted rehabilitation up to this point. Another deficiency lies in the fragmented and incomplete data provided in the available studies, despite the fact that cognitive and motor rehabilitation plays a key role in the improvement of patients with MS [7].
The purpose of our study was therefore to build on the previously mentioned need for objective research, while trying to suppress all the known defects from previous studies that lead to unproductive/misleading results. Therefore, the initial requirement was to produce a study with a significant number of patients allowing for a discernible statistical evaluation, while, at the same time, allowing for a wide range of testing techniques to be used. The cited studies always included either a low number of patients, which allowed for only very unproductive quantitative evaluation [17] or studies with a large number of patients which allowed for the utilization of only a limited amount of testing material, which influenced their conclusions – the use of only self-assessment methods does not show the condition of the patient objectively, but only reflects his/her own assessment of the situation, which can be influenced, for example, by the fact that someone is interested in the patient [3]. Thus, by combining the need for statistical evaluation and the possibility of using a wide range of testing material, we consider that we addressed these shortcomings and intended to enroll approximately 20–30 patients in both groups.
Among other factors, the frequency and duration of training sessions were shown to have a major impact on the effectiveness of the training. For example, training only once per week leads to the improvements in only some of the features. Likewise, a study by Tesar et al showed significant improvements in executive function and spatial-constructional abilities, but no significant improvements in memory or fatigue values [18]. Furthermore, Rosti-Otajärvi et al showed a positive effect of the rehabilitation of cognitive functions on perceived cognitive impairment. Decreased levels of depression and fatigue were also noted. However, these results were verified only through the use of self-assessment scales [19]. On the other hand, training performed 3–6 times per week for 6–12 weeks showed the improvement that manifested in a number of cognitive functions [20]. Some patients even experienced improvements in the activities of daily living [3] and improvements in emotions [20–22]. After the systematic reviews of Mitolo et al [5] and Magalhães et al [9], we have chosen to train 4 times per week with 30 min per session for 8 consecutive weeks. Therefore, we had 32 training sessions overall that took place on predetermined days and followed a specific training plan. The duration of the training was carefully chosen to allow for the notation of positive and verifiable results. Some of the previous studies used short or low intensity training, e.g., no improvement in cognitive performance, but a positive effect on cognitive deficit was found [8]. The authors believe that training once a week for 60 min cannot lead to objectively measurable improvement, but that only the subjective perception of patients may be positively influenced by such a training. Comparing and examining individual studies, we decided on a target training time of approximately 4 times per week for at least 2 months. Individual studies use different computer training methods. For example study performed by Messinis et al summarizes positive results when using the RehaCom computer program [23]. Patients perceived this training very positively. Based on the comparison and examination of individual studies, we decided the target training time of approximately 4 times per week for at least 2 months. A shorter training time appears to be not so effective, while longer training times lead to increased fatigue and memory concentration problems in patients with MS.
The use of the Happy Neuron Brain Jogging software was proven to be very useful in fine tuning the patients and for the adherence to the training parameters away from the sessions with the therapist. Patients considered the software as user-friendly. They received the instruction to perceive this training as entertainment rather than rehabilitation and as an opportunity to actively participate in the improvement of their condition. This recommendation was accepted without any difficulty.
The training results were recorded immediately after the end of the training session with the help of a specially developed web program. In the event of a breakdown in the prescribed training program, the patient was contacted. This ensured a strict adherence to the prescribed training conditions. The Happy Neuron Brain Jogging training program was chosen for its acceptable price for patients and also because it is available in the Czech version.
A great deal of attention was paid to the creation of the selected and evaluated group. We intended to compile a group focused on trained functions, while trying to be sensitive enough to recognize the changes in patients with mild cognitive deficit. Based on the review of previous studies [10], the testing methods included objective as well as self-assessment (subjective) measures. We also included methods that focused on the changes in the area of emotions. The objective part of this research examined the quantitative indicators, while its subjective part allowed for the elimination of the placebo effect that was measured in the control group. Furthermore, this shows the importance of having a control group – since most of the published studies include a control group, but do not always use it for the elimination of the placebo effect, and the results of the experimental and control groups generally do not differ [24]. Therefore, we included a wide range of testing methods – both objective (RBANS, TMT, HAMD etc.) and subjective (BDI-II, SOS, SP/PP, CFQ).
An important part in the evaluation of the results was to eliminate the practice effect, which occurs in both the experimental and control conditions, and which is also measurable by objective test methods. The elimination of the practice effect measured in the control group from the results of the experimental group shows the actual effect of the measured cognitive training.
Patients did not participate in any other form of cognitive training and did not use cognitive enhancing drugs during this study.
Our study presented statistically significant improvements in patients included in the experimental group, even after the elimination of the practice and placebo effects, while no change was observed in patients in the control group, after the elimination of the practice and placebo effects. As for the RBANS in the selected and evaluated group, the results of the experimental group showed the greatest improvement in the immediate memory and Visuospatial/Constructional coordination, which corresponded with the used Neuron software technology that focused on graphic objects rather than purely verbal objects.
The results of this study demonstrate a positive effect of neuropsychological rehabilitation in patients with MS that participate in regular computer-controlled training, in our case, 4 times per week for 8 consecutive weeks.
Limitations
The efforts to limit the placebo effect and the practice effect recommend the use of three groups of patients: 1. an experimental group; 2. a placebo group that would be kept in some contact with the research team; and 3. a control group that would have no contact with the research team. In this way, more accurate assessment of the training results can be obtained. In this study, the control group would ideally play a role of placebo effect. Nevertheless, the comparison of the results between the placebo and control groups would allow a targeted elimination of the placebo effect from the practice effect. We perceive that the practice effect as the effect that arises during testing and is manifested in both the experimental and control groups should be eliminated as a such. However, this influence of a practice effect should be differentiated from the above-mentioned placebo effect.
This study also did not include follow-up examinations. These would be useful for the demonstration of otherwise sustained effect of the measured improvement in the patient’s cognition.
Conclusion
The results of this study show a positive effect of neuropsychological rehabilitation in patients with MS in the above-mentioned structured training. The results showed improvements in the experimental group in the overall RBANS testing of the group and in other objective techniques. The improvements were shown to be statistically significant – even after the elimination of the practice/placebo effect measured in the control group. The greatest improvement was shown to be in the areas of memory and attention.
To summarize, the rehabilitation of cognitive functions has a positive effect in patients with MS, provided that a certain set of criteria were met, both within the diagnostic process and especially within the training program. The important part lies not only in the frequency of the training, but also in its distribution over time. The training in our study occurred 4-times per week with 30-min sessions. Another important training factor was the choice of software. In this case, Happy Neuron Brain Jogging was used and patients rated this software as user-friendly, interesting and affordable for them. The fact that the software is available in the Czech language is an important factor. From our point of view, the program fulfilled all the requirements for training the specific functions that our study was targeting. Patients received and kept this training on a CD, so that they could continue their training after the termination of the study. The long-term benefit of such a training is a suggestion for further research.
We assume that the positive effect of the rehabilitation program can have a significant impact on the improvement of the quality of life of MS patients and improve their chance of obtaining useful employment. This, however, also needs to be confirmed by studies focused on the quality of life of MS patients.
Ethical principles
All patients provided written informed consent and the study protocol was approved by the ethics committee of the Charles University Faculty Hospital and all methods were performed in accordance with the relevant guidelines and regulations.
Disclosure
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Accepted for review: 25. 12. 2019
Accepted for print: 1. 7. 2020
Luděk Fiala, MD, MBA, PhD
Department of Psychiatry First Faculty of Medicine Charles University
Ke Karlovu 11
120 00 Prague 2
Czech Republic
e-mail: ludek.fi ala@vfn.cz
Zdroje
1. Langdon DW. Cognition in multiple sclerosis. Curr Opion Neurol 2011; 24 (3): 244–249. doi: 10.1097/WCO.0b013e328346a43b.
2. Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008; 7 (12): 1139–1151. doi: 10.1016/S1474-4422 (08) 70259-X.
3. Stuifbergen AK, Becker H, Perez F et al. A randomized controlled trial of a cognitive rehabilitation intervention for persons with multiple sclerosis. Clin Rehabil 2012; 26 (10): 882–893. doi: 10.1177/0269215511434997.
4. Chmelarova D, Ambler Z, Dostal M et al. Cognitive rehabilitation in patients with multiple sclerosis. Cesk Slov Neurol N 2014; 77/110 (6): 677–683.
5. Mitolo M, Venneri A, Wilkinson ID et al. Cognitive rehabilitation in multiple sclerosis: a systematic review. J Neurol Sci 2015; 354 (1–2): 1–9. doi: 10.1016/j.jns.2015.05.004.
6. Prosperini L, Piattella MC, Giannì C et al. Functional and structural brain plasticity enhanced by motor and cognitive rehabilitation in multiple sclerosis. Neural Plast 2015; 2015 : 481574. doi: 10.1155/2015/481574.
7. De Luca J, Nocentini U. Neuropsychological, medical and rehabilitative management of persons with multiple sclerosis. NeuroRehabilitation 2011; 29 (3): 197–219. doi: 10.3233/NRE-2011-0695.
8. Mäntynen A, Rosti-Otajärvi E, Koivisto K et al. Neuropsychological rehabilitation does not improve cognitive performance but reduces perceived cognitive deficits in patients with multiple sclerosis: a randomised, controlled, multi-centre trial. Mult Scler 2014; 20 (1): 99–107. doi: 10.1177/1352458513494487.
9. Magalhães R, Alves J, Thomas RE et al. Are cognitive interventions for multiple sclerosis effective and feasible? Restor Neurol Neurosci 2014; 32 (5): 623–638. doi: 10.3233/RNN-140388.
10. Amato MP, Zipoli V, Portaccio E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci 2006; 245 (1–2): 41–46. doi: 10.1016/j.jns.2005.08.019.
11. Rosti-Otajärvi E, Mäntynen A, Koivisto K et al. Neuropsychological rehabilitation has beneficial effects on perceived cognitive deficits in multiple sclerosis during nine-month follow-up. J Neurol Sci 2013; 334 (1–2): 154–160. doi: 10.1016/j.jns.2013.08.017.
12. Broadbent DE, Cooper PF, FitzGerald P et al. The cognitive failures questionnaire (CFQ) and its correlates. Br J Clin Psychol 1982; 21 (1): 1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x.
13. Dragomirecka E, Lenderking WR, Motlova L et al. A brief mental health outcomes measure: translation and validation of the Czech version of the Schwartz Outcomes Scale-10. Qual Life Res 2006; 15 (2): 307–312. doi: 10.1007/s11136-005-1389-y.
14. Bagby RM, Ryder AG, Schuller R et al. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry 2004; 161 (12): 2163–2177. doi: 10.1176/appi.ajp.161.12.2163.
15. Man DW, Soong WY, Tam SF et al. Self-efficacy outcomes of people with brain injury in cognitive skill training using different types of trainer–trainee interaction. Brain Inj 2006; 20 (9): 959–970. doi: 10.1080/02699050600909789.
16. Giacomini PS, Arnold DL. Non-conventional MRI techniques for measuring neuroprotection, repair and plasticity in multiple sclerosis. Curr Opin Neurol 2008; 21 (3): 272–277. doi: 10.1097/WCO.0b013e328300525b.
17. Brissart H, Leroy M, Morele E et al. Cognitive rehabilitation in multiple sclerosis. Neurocase 2013; 19 (6): 553–565. doi: 10.1080/13554794.2012.701644.
18. Tesar N, Bandion K, Baumhackl U. Efficacy of a neuropsychological training programme for patients with multiple sclerosis – a randomised controlled trial. Wien Klin Wochenschr 2008; 117 (21–22): 747–754. doi: 10.1007/s00508-005-0470-4.
19. Rosti-Otajärvi EM, Hämäläinen PI. Neuropsychological rehabilitation for multiple sclerosis. Cochrane Database Syst Rev 2014; (2): CD009131. doi: 10.1002/14651858.CD009131.pub3.
20. Brenk A, Laun K, Haase CG. Short-term cognitive training improves mental efficiency and mood in patients with multiple sclerosis. Eur Neurol 2008; 60 (6): 304–309. doi: 10.1159/000157885.
21. Mattioli F, Stampatori C, Zanotti D et al. Efficacy and specificity of intensive cognitive rehabilitation of attention and executive functions in multiple sclerosis. J Neurol Sci 2010; 288 (1–2): 101–105. doi: 10.1016/j.jns.2009.09.024.
22. Parisi L, Rocca MA, Mattioli F et al. Changes of brain resting state functional connectivity predict the persistence of cognitive rehabilitation effects in patients with multiple sclerosis. Mult Scler 2014; 20 (6): 686–694. doi: 10.1177/1352458513505692.
23. Messinis L, Nasios G, Kosmidis MH. Efficacy of a computer-assisted cognitive rehabilitation intervention in relapsing-remitting multiple sclerosis patients: a multicenter randomized controlled trial. Behav Neurol 2017; 2017 : 5919841. doi: 10.1155/2017/5919841.
24. Solari A, Motta A, Mendozzi L et al. Computer-aided retraining of memory and attention in people with multiple sclerosis: a randomized, double-blind controlled trial. J Neurol Sci 2004; 222 (1–2): 99–104. doi: 10.1016/ j.jns.2004.04.027.
Štítky
Dětská neurologie Neurochirurgie NeurologieČlánek vyšel v časopise
Česká a slovenská neurologie a neurochirurgie

2020 Číslo 4
-
Všechny články tohoto čísla
- Editorial
- Cytotoxic lesions of the corpus callosum (CLOCCs)
- Radial nerve injury associated with humeral shaft fracture
- It is evident when to make a surgery for lumbar disc herniation?
- Current diagnostics of secondary progressive form of multiple sclerosis and its treatment with siponimod
- Multiple sclerosis – behind the immunity curtains
- Airway clearance in patients with Parkinson‘s disease – overview and possibilities of physiotherapeutic intervention
- Clinical and social predictors of quality of life in children and young adults with autism spectrum disorder
- Safety of carotid endarterectomy in relation to the timing after ischemic stroke
- Glatirameracetate – the treatment of multiple sclerosis monitored in the ReMuS Registry
- Intensive computer-assisted cognitive rehabilitation in persons with multiple sclerosis – results of a 12-week randomized study
- Efficacy and safety of emergent microsurgical embolectomy in patients with acute ischemic stroke after the failure of intravenous thrombolysis and mechanical thrombectomy – a systematic review protocol
- Impact of the COVID-19 pandemic on sleep medicine in the Czech Republic and Slovakia
- The prevalence and characteristics of epilepsy in patients with relapsing-remitting multiple sclerosis treated with disease-modifying therapy
- Moyamoya syndrome associated with polycystic kidney disease – a rare case report and literature review
- Souběh dvou oportunních infekcí jako první projev HIV
- Dropped head syndrome in patient with progressive bulbar palsy
- Carotid body paraganglioma, a very rare pediatric tumor
- Využití kvantitativní MR venografie v indikaci stentingu stenózy žilního splavu
- Transvenous embolization of a ruptured brain arteriovenous malformation
- CGRP monoclonal antibodies in the treatment of migraine – indication criteria and therapeutic recommendations for the Czech Republic
- Recenze knih
- 2020 AAN Highlights Dlouhodobá data o účinnosti deplece CD20+ B-buněk v léčbě RS
- 2020 AAN Highlights Jak mění malá molekula průběh spinální svalové atrofie?
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle
- It is evident when to make a surgery for lumbar disc herniation?
- CGRP monoclonal antibodies in the treatment of migraine – indication criteria and therapeutic recommendations for the Czech Republic
- Cytotoxic lesions of the corpus callosum (CLOCCs)
- Dropped head syndrome in patient with progressive bulbar palsy
