Pattern of Postural Changes after Symmetric Neck Muscle Vibration
Vzorec posturálnych zmien v súvislosti so symetrickou vibráciou krčných svalov
Úvod:
Cieľom práce bolo štúdium reflexov zo svalov dolných končatín a očných pohybov, v kontexte posturálnych synergií, ktoré boli evokované vibráciou zadného krčného svalstva.
Metodika:
Vyšetrili sme 12 zdravých osôb. Zaznamenávali sme elektromyografiu (EMG) z dolných končatín, náklonovú dráhu (posturografia), pozíciu segmentov tela a elektrookulografiu v náväznosti na pulzy vibrácie zadných krčných svalov, ktoré trvali 2 sekundy. Hodnotili sa spriemernené odpovede.
Výsledky:
Posturálna odpoveď na vibráciu zadného krčného svalstva pozostáva z krátkeho fázického posunu centra oporných síl dozadu, na ktorý nadväzuje dlhšie trvajúci posun dopredu. Telo sa počas tejto fázy pohybuje dopredu. Prvá fáza začína svalovými reakciami, ktorých latencie sú porovnateľné s posturálnymi reflexami vyvolanými galvanickou stimuláciou vestibulárneho aparátu, ktoré sú známe z minulosti (krátko - a strednelatenčné reflexy). Podkladom druhej fázy sú dlhšie trvajúce zmeny svalovej aktivácie. Narozdiel od zdravých probandov, už publikované práce ukázali, že pacienti s bilaterálnou vestibulárnou afunkciou majú len druhú časť odpovede a aj tá má len „lokálny“ charakter (extenzia hlavy). Väčšina probandov mala počas svalovej vibrácie pomalý fázický pohyb očí smerom dolu.
Záver:
Naša práca osvetľuje úlohu krčnej propriocepcie v regulácii vzpriameného postoja, ktorej správne fungovanie vitálne závisí na integrite vestibulárneho systém. Celkový vzorec pohybov tela a očí nie je možné jednoducho vysvetliť vestibulo-okulárnymi a cerviko-okulárnymi reflexami. Predpokladáme, že sú skôr pozostatkom tonických krčných reflexov, ktorá sa objavujú u ľudských dojčiat a zvierat.
Kľúčové slová:
propriocepcia – svalová vibrácia – posturografia – regulácia postoja – zadné krčné svalstvo
Authors:
P. Valkovič 1–3; S. Krafczyk 1; J. Levin 1; K. Bötzel 1
Authors‘ workplace:
Dept. of Neurology, Ludwig-Maximilians University, Munich, Germany
1; 2nd Department of Neurology, Comenius University, Bratislava
2; Laboratory of Motor Control, Institute of Normal and Pathological Physiology, Slovak Academy of Sciences, Bratislava
3
Published in:
Cesk Slov Neurol N 2012; 75/108(3): 344-350
Category:
Original Paper
Overview
Introduction:
The aim of this study was to explore, in the context of postural synergies, reflexes from lower limb muscles and ocular movements induced by posterior neck muscle vibration.
Methods:
We examined 12 healthy subjects. We recorded lower limb EMG, sway path (posturography), the position of body egments, and the eye movements (electrooculography) during 2 seconds lasting pulses of posterior neck muscle vibration. The response means were evaluated.
Results:
Postural response to posterior neck vibration consists of a short phasic backward shift of the centre of foot pressure, which initiates a more prolonged anterior shift. The body moves forward during this second phase. The first phase is initiated by muscular reactions comparable to previously described postural reflexes elicited by galvanic vestibular stimulation (short and medium latency reflexes). The second phase is based on longer-lasting changes to muscular activation. In contrast to healthy subjects, previous studies showed that patients with total vestibular loss produce the second response only. Moreover, this reaction is “local” (head extension). In the majority of subjects, a slowly downward drifting movement of the eyes was observed during neck muscle vibration.
Conclusion:
Our study elucidates the role of neck proprioception in upright stance control, the correct functioning of which vitally depends on the integrity of the vestibular system. Oculomotor responses observed in this study were more probably part of a general postural synergy and not of the cervico-ocular reflex, and could be interpreted as a remnant of the tonic postural reflex.
Key words:
proprioception – muscle vibration – posturography – postural control – posterior neck muscles
Introduction
Proprioceptive input from the neck contributes to reflexive coordination of the eye, head, and body position as well as to the perception of the body and of targets in the extrapersonal space. Disturbance of these signals may cause dizziness with imbalance (see [1] for a review). The most important source of proprioceptive information originates from the deep neck muscles that are extensively furnished with muscle spindles [2]. Experimentally, these elements can be stimulated by muscle vibration that selectively stimulates primary endings of the muscle spindles, having the same effect as muscles stretching [3]. If applied unilaterally to the posterior neck muscles, the CNS interprets this as “erroneous” signal and shifts the subjective “straight ahead” towards the side of the vibrated neck muscle [4] as well as it elicits the illusory motion of a target light in the dark towards the contralateral side [5].
Bilateral vibration of posterior neck muscles in standing humans causes an erroneous perception of backward inclination of the body to which the postural reaction is leaning forward [6,7]. This reaction vitally depends on the integrity of the vestibular system. Its absence in patients with vestibular loss [8] supports previous evidence that neck proprioception is interpreted in the context of vestibular signals evoked by head movement [9,10]. Electromyographic (EMG) responses of lower limb muscles occur with a latency of 70–100 ms after an onset of neck muscle vibration [11]. It is still unclear whether these reflexes are unspecific or crucial for eliciting body sway, because vibration-induced muscle reflexes, sway path, and body movements have not been measured simultaneously. Furthermore, associated eye movements have not been studied yet. They may reveal important insights into the physiology of vibration-induced postural reactions. It is possible that these vestibulo-spinal responses could occur without involvement of eye movements. However, an association between eye movements and postural destabilization has recently been described [12]. In addition, the direction of sway induced by neck vibration depends on gaze direction [13]. To be able to compare leg muscle EMG with posturographic data, we recorded lower limb EMG, sway path (posturography) and the position of body segments in the same setting during neck muscle vibration. Furthermore, eye movements were recorded using electro-oculography.
Methods
Subjects
Twelve healthy volunteers (seven men, ages 20–35 years) participated in two test series (see below) after giving their informed consent according to the Declaration of Helsinki. Participants had no neurological or musculoskeletal disorder. In addition, they were under influence of no neurotropic substance that could affect balance or other assessed domains. The study was approved by a local ethics committee.
Muscle vibration
Vibration was applied bilaterally with two identical cylindrical vibrators containing a DC micromotor (Faulhaber, type 2842C, Germany) with eccentric weights on both ends of the axes. The motors operated at 20 V induced a 70 Hz vibration with 1.4 mm peak amplitude. The cylinders containing the motors were mounted on a flat plate (7.0 × 2.0 cm) positioned so that their centers were approximately 5 cm above the spinous process of the 7th cervical vertebra and 4 cm lateral from the midline. In this position, they stimulated lower sections of the splenius muscle and upper sections of the trapezius muscle. Vibrators were taped onto the skin and secured with an elastic dressing around the dorsal neck area, the chest, and under the armpits. An accelerometer was placed on the vibrator to assess electromechanical delay between a trigger signal (computer command) and physical vibration stimulus. The very first mechanical event occurred 23 ms after the trigger signal; the first peak (25% of the first rotation) was achieved at 31 ms when it had 20% of steady state peak-to--peak amplitude. This moment was arbitrarily taken as the onset of vibration, and 31 ms (trigger delay) were always subtracted when calculating postural, positional, and EMG response latencies. The first revolution of the motors was completed 44 ms after a trigger impulse.
Experimental setup and data evaluation
A series of two separate studies were performed. During the experiments, subjects were standing in an upright position and had their eyes closed. 2-second-long vibration pulses were applied at random intervals (duty cycle 5–15 s).
Study 1
To investigate changes to the center of foot pressure (COP) during 2-s-long vibration pulses, the subjects stood upright on a posturographic platform with their eyes closed (Kistler, type 9281B, Switzerland). The heels of their feet were touching each other and the feet made an angle of 30°. Arms were crossed on the chest. Ten pulses of vibration were applied. The anterior-posterior (a-p) COP path was sampled at 40 Hz and the mean calculated for each subject (see below). Amplitudes and latencies of each subject‘s curves were defined by inspection. During the same experiments, movements of several body segments were recorded using an ultrasonic device (Zebris, type CMS 70P, Germany). This system continuously calculates 3-D spatial positions of small ultrasound emitting markers that were firmly taped to moving body parts. Four markers were placed on the left site of the body: 1. head marker, over the zygomatic arch; 2. shoulder marker, over the greater tubercle of the humerus; 3. hip marker, over the anterior superior spine of the iliac bone; and 4. knee marker, over the lateral epicondyle of the femur. Three-dimensional coordinates of the four markers were sampled at a frequency of 40 Hz each. From these data, time-dependent position of the markers in the sagittal plane was depicted as well as the angles between the four body segments (shank, thigh, trunk, and head) and the horizontal plane. Segments were defined as lines connecting the corresponding markers. The segment between shoulder marker and head marker was termed “head” and thus also covered the neck. To calculate the angle between shank and the horizontal plane, a stationary foot marker was automatically added by the software at a distance from the knee, scaled to the height of the subject. The means (10 responses) for individual subjects were evaluated in the same way as the posturographic traces.
Study 2
To record electromyographic activation of leg muscles and eye movements during 2-s-long vibration pulses, the subject stood upright with their eyes closed. Since postural EMG activation can be better seen in pre-activated muscles, the subject leaned forward (in order to activate the gastrocnemius and biceps femoris muscles) and backward (activation of the anterior tibial and quadriceps muscles) in separate trials. Each trial was repeated once, resulting in 40 vibration pulses altogether. In this setting, electromyographic (EMG) activity of four leg muscles was recorded bilaterally: anterior tibial, medial gastrocnemius, quadriceps, and biceps femoris. Two silver cup electrodes filled with electrode gel were attached to the muscle bellies (3 cm apart), and signals were recorded in a bipolar fashion. (Amplification: gain 1,000, filter 10–1,000 Hz). These data were digitized (2,000 Hz) and continuously stored on a digital disk together with trigger impulses. EMG signals were rectified off-line after high-pass filtering to eliminate baseline shifts and then averaged for each subject (separately for leaning forward and backward). The time window for averaging ranged from 200 ms before the trigger to 1,900 ms afterward. To detect any change of muscular activity during vibration, the mean EMG amplitude during three 100-ms-intervals (–100 to 0 ms; 900 to 1,000 ms; 1,800 to 1,900 ms) was computed for each subject and evaluated statistically (ANOVA for repeated measures). Vertical and horizontal eye movements were recorded with electro-oculography (EOG) using Ag/AgCl electrodes (gain 1,000; filters 0.1–100 Hz) while the eyes were closed. The EOG was carefully calibrated. The evaluation included measurement of vertical slow phase velocity from 1,200 ms before the onset of vibration until 2,000 ms after the onset. These measurements were performed by automatically fitting a line to eye movement segments not containing saccades [14]. This resulted in 2–15 segments for each vibration trial, the steepness of which (slow phase velocity) was stored together with the time at which this steepness was measured. These measurements as well as all other evaluations were made by software programmed in MATLAB (The MathWorks Inc., Natick, MA, USA).
Results
Study 1, posturography and measurements of body position
After an onset of neck vibration, a slight transient backward shift of the COP beginning at 106 ms (L1 in Fig. 1 left) was recorded for all subjects that reverted to a sustained forward COP movement starting at 359 ms (L2 in Fig. 1 left, Tab. 1). The amplitude of the first backward shift was 4.5 mm (S.D. 3.1 mm; A1 in Fig. 1 left), the maximum amplitude of the forward COP displacement occurring at latency L4 was 9.2 mm (S.D. 8.9 mm; marker ‚A2‘, Fig. 1 left).

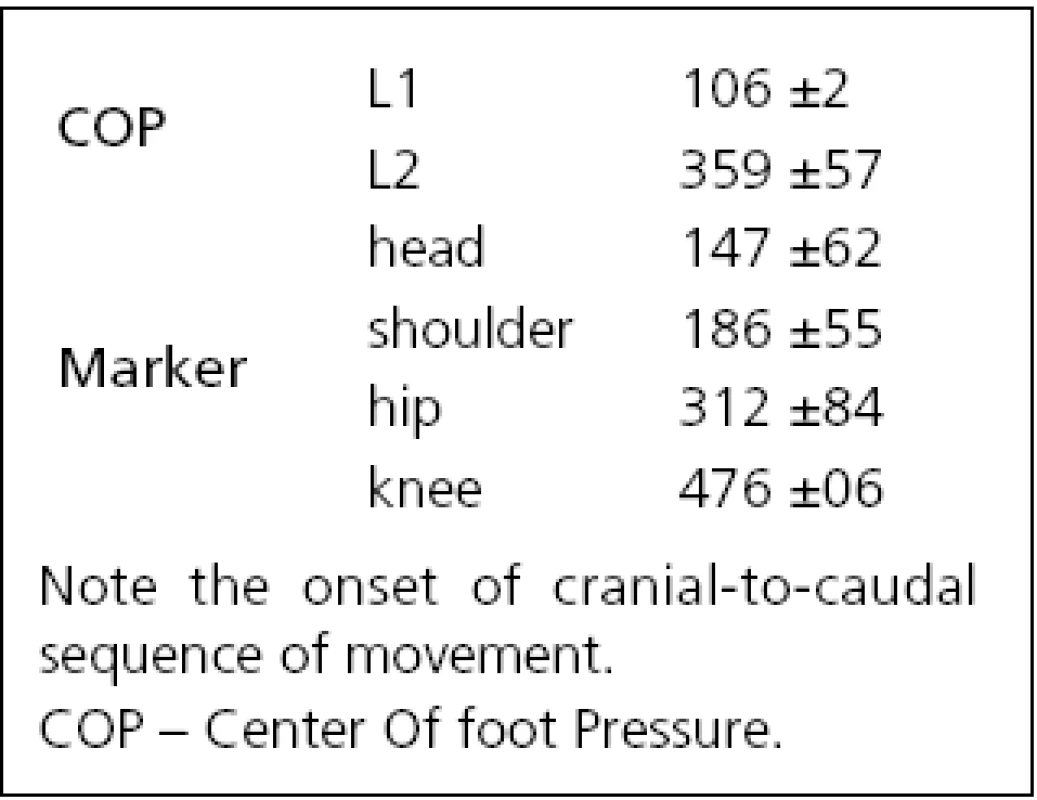
The positional data from the ultrasound markers showed a forward movement of all body segments for all subjects with the exception of a minor initial backward movement of knee and hip in six of 12 subjects. The onset of the forward movement, as defined on the individual traces of subjects, varied from 147 ms (head) to 476 ms (knee) i.e., the head led the body (Tab. 1, Fig. 1 middle). During a 2-s vibration period, as the whole body bent forward in a uniform manner, angles between the individual body segments and horizontal plane decreased slowly by –2 degrees (Fig. 1 right). The head segment reacted in conjunction with the other segments and never showed an “individual” reaction, albeit the stimulus was applied only at this segment.
Study 2, electromyography and electro-oculography
When the subject leaned forward, there was a reduction of activity in the gastrocnemius and biceps femoris muscles beginning at 70 ms and lasting for 500 ms. There was a biphasic pattern at the beginning of reduced EMG activity, with troughs at 90 and 150 ms (Fig. 2). Latency difference of these troughs between thigh and shank muscles was about 2.5 ms. Thereafter, a slow increase in EMG activity was noted in these two muscles as the body moved further forward. Corresponding quadriceps and anterior tibial muscles showed little activity during forward leaning and two small peaks at 90 and 150 ms, at the time when the two troughs were seen in the posterior muscles.

When the subject leaned backward, the quadriceps and tibialis anterior muscles were pre-activated. Initially, they showed increased activity with two small peaks at 90 and 150 ms, followed by a slow decrease in muscular activity. In this setting, the biceps femoris as well as the gastrocnemius muscles showed comparably little activity.
An ANOVA for repeated measures of the mean amplitudes at –100 to 0 ms, 900 to 1,000 ms, and 1,800 to 1,900 ms showed a significant effect of “time” for all muscles during forward leaning and for flexor muscles during backward leaning only.
The EOG traces of six subjects showed a clear down-drift eye movement with frequent saccades upward resembling an upbeat nystagmus. Four subjects showed discrete, transient down-drift eye movements, and two subjects had a slight up--drift eye movement. Mean slow phase velocities of the down-drift eye movements were in the range of 2 to 6 deg/s and differed between the subjects (Figs 3 and 4). In some subjects, down-drifting eye movements could also be seen before the onset of the actual stimulus (measured from 200 ms before the beginning of the vibration).


Discussion
In this study, we aimed to answer three questions: what is the correlation between postural reactions and muscular reflexes caused by vibration, which pattern of postural changes can be elicited by this stimulus and does this stimulus evoke eye movements detectable by means of electro-oculography?
Our posturographic measurements showed that the forward movement of the body caused by neck vibration consists of two phases: first, the COP shifts to the heels (from 106 to 359 ms), and, due to this shift, the body falls forward. During this fall, the COP moves to the toes, causing the fall to terminate by achieving equilibrium of the body in a forward, bent position. The first component can be caused by either contracting the tibialis anterior muscle or releasing the gastrocnemius activation. Exactly these phenomena were observed in the EMG responses, i.e., when leaning backwards, the tibialis anterior muscle increased its activity starting at 70 ms, whereas during forward leaning, the gastrocnemius released its force at the same time. In our recordings, these EMG reflexes have a mirror-image like polarity in the anterior and posterior leg muscles. The basic pattern of these vibration-induced leg muscle responses was first described by Andersson and Magnusson in 2002 [11]. The fast EMG components at 90 and 150 ms seem to represent the short and medium latency components described after galvanic vestibular stimulation [15–17]. These latencies are in the same range as the fast vestibulospinal reflexes elicited by loud tones stimulating the sacculus [18] or proprioceptive stimulation by tapping the head or chest with a reflex hammer [19]. Our current data provide evidence that these reflexes are not merely unspecific epiphenomena but represent muscular activation that causes the first part of synergy (shifting the COP to the heels), subsequently leading to forward fall of the body. Due to the inertia of the body, the second part of the synergy is slower and is represented by statistically significant activation changes of leg muscles during the 2-s-vibration period.
The head started to move forward at 147 ms after the onset of the stimulus. The angle between different body segments and the horizontal plane changed by the same degree, i.e., inter-segmental angles remained stable and the body moved as an inverted pendulum with the ankle joint as pivot. The posterior neck muscles, to which vibration was applied, did not seem to react at all. This is indicated by the fact that the head segment showed no other inclination compared to the other body segments. Thus, the posterior neck muscles did not react on a “local” basis with a contraction, as would be the case if the vibration signal was interpreted by local stretch reflex mechanisms as is the case in subjects with vestibular loss [8]. Similarly to Lekhel et al [8], we did observe “local” reaction in our previous study [20]. Contrary to normal subjects, posterior neck vibration pulses led to consistent backward extension of the head in all three patients with total vestibular loss. This confirmatory finding emphasizes the role of neck proprioception in upright stance control that also vitally depends on the integrity of the vestibular system.
In the majority of subjects, the forward bending movement of the body was accompanied by a rather constant downward eye movement that was interrupted by upward saccades, resulting in an upbeat nystagmus. EOG allowed us to measure these eye movements even when the subject had their eyes closed. Down-drifting eye movements were also frequently seen before a new stimulus began, i.e. they outlasted the preceding stimulation. Although all subjects showed the described postural changes, direction and velocity of eye movements differed among the subjects. This is similar to the perceptual phenomena induced by neck vibration that are also reported to be inter-individually different [13,21]. Down--drifting eye movements evoked by bilateral posterior neck muscle vibration have been previously seen in subjects with fixed head [22,23], whereas in animal experiments, this stimulus was reported to cause up-drifting eye movements [24]. The latter direction would be compatible with a cervico-ocular reflex compensating for a distension of posterior neck muscles. Considering the difference in locomotion (quadrupedal vs bipedal), it seems possible that these reflexes are different in man and monkey.
The eye movements observed in our experiments outlasted the stimulus. Experiments using unilateral vibration to investigate the effects on walking in place have shown that the effect of neck vibration can outlast the actual stimulus: after the onset of stimulation a delay of up to 10s was noted before subjects began to rotate, and rotation continued for some time after the end of stimulation [25,26]. The authors of the latter study attributed this delay to the necessity to integrate neck proprioceptive inflow into the neural circuits’ activity responsible for construction of spatial references.
Thus, besides the fast postural reflexes seen on the EMG traces of every subject, there seems to be at least one other physiological process with longer time constants or possibly integrator capacity. We assume that it is responsible for the recorded eye movements and possibly also for the perception of the assumed body position. However, the latter two processes may be broken down further since dissociation between vibration-induced eye movements and perceptual phenomena has been reported and taken as evidence for separate mechanisms responsible for these phenomena [21].
We propose that the observed processes should be interpreted with a model that suggests that an intermediate level of sensory processes exists above the low level of peripheral reflexes. The position of the body is reconstructed in ‚body-in--space‘ coordinates [27,28]. This is achieved by subtracting intersegmental proprioceptive signals of the body from the signals of the vestibular organ. This concept is substantiated by experimental evidence: in a cat, vestibular and neck proprioceptive signals converge in the lateral vestibular nucleus and in the reticular medullary nucleus on neurons of the vestibulo - and reticulo-spinal tracts, respectively [29]. In monkeys, a convergence of proprioception and vestibular information has been observed in the cerebellar vermis and fastigal nucleus [30]. In these recordings, vestibular information is represented in body-centered rather than head-centered coordinates. Thus, in our experiments, a postural reaction occurs even without direct vestibular stimulation, because of the sum of vestibular and proprioceptive neck signal changes. This explanation is basically identical with the one proposed by Lekhel and colleagues [8]. However, we would formulate it so as to avoid the possibility of interpreting these reactions as being consciously initiated (interpretation of the body leaning backwards). Conscious processes can be excluded on the basis of the latency of the observed reactions.
We interpret the oculomotor responses observed in our study as part of a general postural synergy and not on the basis of a cervico-ocular reflex (COR). The COR would cause the eyes to move opposite to the erroneously perceived head movement, i.e. upwards. There is some controversy about the direction of the COR that has, in the horizontal plane, a compensatory (counter-to-head rotation) tonic component and it has an anticompensatory phasic component effective only at very low velocities [29]. Data on vertical COR have been reported for rabbits [31] but, to our knowledge, are lacking for primates. A vestibulo-ocular reflex can also be excluded, since it would cause upward eye movements during forward head rotation. Thus, the posturo-ocular synergy we observed could be interpreted as a remnant of a tonic postural reflex that Magnus [32] described in a cat.
Conclusion
In our study with posterior neck vibration we showed that the neck vibration-caused forward movement of the body consists of two phases. The first and very rapid reaction caused by EMG activity in lower limb muscles is mediated by vestibulospinal reflex. The second, more complex synergy, reflects coupled engagement of all body segments and starts by head movement. In contrast to normal subjects, previous studies showed that patients with total vestibular loss produce the second response only. Moreover, this reaction is not “contextual” but “local” (head extension). In this context, our study elucidates the role of neck proprioception in upright stance control, correct functioning of which vitally depends on the integrity of the vestibular system. With respect to clinical practice, our data partially explain why some individuals with cervical functional block (and/or pain syndrome) suffer from dizziness (so called “cervical vertigo”) but others do not [1]. We hypothesize that those with cervical block and dizziness suffer from clinically relevant or subclinical vestibular hypofunction that is unmasked if cervical proprioceptive information is no more relevant. In the situation of intact vestibular system, it correctly detects that neck proprioceptive afferent input is false (modified and sustained firing of IA afferent fibers). It does not rely on its information and must react properly in the context of all body orientation in space.
Oculomotor responses observed in this study were more probably a part of a general postural synergy and not a cervico-ocular reflex (COR) and could be interpreted as a remnant of the tonic postural reflex.
Acknowledgements
We are grateful to U. Büttner for helpful comments on the manuscript. We wish to thank J. Benson for skillful editorial help.
Supported by the Scientific Grant Agency of the Slovak Ministry of Education and the Slovak Academy of Sciences (project 1/0070/11).
Assoc. Prof. Peter Valkovič, MD, PhD
2nd Department of Neurology
School of Medicine, Comenius University
Limbová 5
83305 Bratislava
e-mail: Peter.Valkovic@gmail.com
Accepted for review: 11. 7. 2011
Accepted for print: 14. 11. 2011
Sources
1. Brandt T, Bronstein AM. Cervical vertigo. J Neurol Neurosurg Psychiatry 2001; 71(1): 8–12.
2. Richmond FJ, Bakker DA. Anatomical organization and sensory receptor content of soft tissues surrounding upper cervical vertebrae in the cat. J Neurophysiol 1982; 48(1): 49–61.
3. Roll JP, Vedel JP, Ribot E. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res 1989; 76(1): 213–222.
4. Strupp M, Arbusow V, Dieterich M, Sautier W, Brandt T. Perceptual and oculomotor effects of neck muscle vibration in vestibular neuritis. Ipsilateral somatosensory substitution of vestibular function. Brain 1998; 121(4): 677–685.
5. Popov KE, Lekhel H, Faldon M, Bronstein AM, Gresty MA. Visual and oculomotor responses induced by neck vibration in normal subjects and labyrinthine-defective patients. Exp Brain Res 1999; 128(3): 343–352.
6. Lund S. Postural effects of neck muscle vibration in man. Experientia 1980; 36(12): 1398.
7. Kavounoudias A, Gilhodes JC, Roll R, Roll JP. From balance regulation to body orientation: two goals for muscle proprioceptive information processing? Exp Brain Res 1999; 124(1): 80–88.
8. Lekhel H, Popov K, Bronstein A, Gresty M. Postural responses to vibration of neck muscles in patients with uni - and bilateral vestibular loss. Gait Posture 1998; 7(3): 228–236.
9. Roll JP, Vedel JP, Roll R. Eye, head and skeletal muscle spindle feedback in the elaboration of body references. Prog Brain Res 1989; 80 : 113–123.
10. Horak FB, Shupert CL, Dietz V, Horstmann G. Vestibular and somatosensory contributions to responses to head and body displacements in stance. Exp Brain Res 1994; 100(1): 93–106.
11. Andersson G, Magnusson M. Neck vibration causes short-latency electromyographic activation of lower leg muscles in postural reactions of the standing human. Acta Otolaryngol 2002; 122(3): 284–288.
12. Glasauer S, Schneider E, Jahn K, Strupp M, Brandt T. How the eyes move the body. Neurology 2005; 65(8): 1291–1293.
13. Ivanenko YP, Grasso R, Lacquaniti F. Effect of gaze on postural responses to neck proprioceptive and vestibular stimulation in humans. J Physiol 1999; 519(1): 301–314.
14. Bonnet C, Hanuska J, Dombrowski A, Růžička E. Eye movement examination in neurological practice. Cesk Slov Neurol N 2011; 74/107(5): 518–526.
15. Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res 1993; 94(1): 143–151.
16. Fitzpatrick R, Burke D, Gandevia SC. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol (Lond) 1994; 478(2): 363–372.
17. Watson SR, Colebatch JG. EMG responses in the soleus muscles evoked by unipolar galvanic vestibular stimulation. Electroenceph Clin Neurophysiol Electromyogr Mot Contol 1997; 105(6): 476–483.
18. Bötzel K, Kolev OI, Brandt T. Comparison of tap-evoked and tone-evoked postural reflexes in humans. Gait Posture 2006; 23(3): 324–330.
19. Bötzel K, Feise P, Kolev OI, Krafczyk S, Brandt T. Postural reflexes evoked by tapping forehead and chest. Exp Brain Res 2001; 138(4): 446–451.
20. Valkovič P, Krafczyk K, Bötzel K. Neck proprioceptive influence on postural control: a study using muscle vibration. Eur J Neurol 2006; 13 (Suppl 2): 144.
21. Seizova-Cajic T, Sachtler WL, Curthoys IS. Eye movements cannot explain vibration-induced visual motion and motion aftereffect. Exp Brain Res 2006; 173(1): 141–152.
22. Lennerstrand G, Han Y, Velay JL. Properties of eye movements induced by activation of neck muscle proprioceptors. Graefes Arch Clin Exp Ophthalmol 1996; 234(11): 703–709.
23. Gomez S, Patel M, Magnusson M, Johansson L, Einarsson EJ, Fransson PA. Differences between body movement adaptation to calf and neck muscle vibratory proprioceptive stimulation. Gait Posture 2009; 30(1): 93–99.
24. Corneil BD, Andersen RA. Dorsal neck muscle vibration induces upward shifts in the endpoints of memory-guided saccades in monkeys. J Neurophysiol 2004; 92(1): 553–566.
25. Bove M, Courtine G, Schieppati M. Neck muscle vibration and spatial orientation during stepping in place in humans. J Neurophysiol 2002; 88(5): 2232–2241.
26. Karnath HO, Reich E, Rorden C, Fetter M, Driver J. The perception of body orientation after neck-proprioceptive stimulation. Effects of time and of visual cueing. Exp Brain Res 2002; 143(3): 350–358.
27. Mergner T, Schweigart G, Botti F, Lehmann A. Eye movements evoked by proprioceptive stimulation along the body axis in humans. Exp Brain Res 1998; 120(4): 450–460.
28. Pawlak-Osińska K, Kaźmierczak H, Kaźmierczak W. Postural reflexes in conditions of visual disturbance. Cesk Slov Neurol N 2011; 74/107(6): 669–674.
29. Pompeiano O, Manzoni D, Srivastava UC, Stampacchia G. Convergence and interaction of neck and macular vestibular inputs on reticulospinal neurons. Neuroscience 1984; 12(1): 111–128.
30. Kleine JF, Guan Y, Kipiani E, Glonti L, Hoshi M, Buttner U. Trunk position influences vestibular responses of fastigial nucleus neurons in the alert monkey. J Neurophysiol 2004; 91(5): 2090–2100.
31. Barmack NH, Nastos MA, Petorossi VE. The horizontal and vertical cervico-ocular reflexes of the rabbit. Brain Res 1981; 224(2): 261–278.
32. Magnus R. Some results of studies in the physiology of posture. The Lancet 1926; 211 : 531–536.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2012 Issue 3
-
All articles in this issue
- Surgical Treatment of Rheumatoid Lesion at Craniocervical Junction
- Conformation Specific Antibodies and Diagnosis of Prion Diseases
- Low Back Pain and Depression – Mutual Relationship
- Neurosyphilis
- Diagnosis and Treatment Options for Niemann-Pick Disease Type C
- An Association between Matrix Metalloproteinase-2 and TIMP-2 +853G/A Gene Polymorphisms and Multiple Sclerosis
- Neurophysiological Diagnosis of the Ulnar Nerve Damage at the Elbow
- Extended Transnasal Endoscopic Approach for Skull Base Tumors and Tumors of the Orbit
- Determination of Cerebellar Dominance from Muscle Tone of the Limbs
- Surgical Treatment of a Tarsal Tunnel Syndrome
- Posterior Interhemispheric Precuneal/Transplenial Approach to Intrinsic Brain Lesions
- Atypical Paraneoplastic Neurological Syndrome – a Case Report
- Bilateral Phrenic Nerve Lesion Manifesting as an Orthopnea – Three Case Reports
- Alzheimer’s Disease Manifesting as Corticobasal Degeneration – Case Report
- Treatment Adherence in Patients with Schizophrenia
- Tau Protein and Anti-Tau Antibodies in Patients with Multiple Sclerosis
- Pattern of Postural Changes after Symmetric Neck Muscle Vibration
- A Dorsal Neurenteric Cyst of the Craniocervical Junction – a Case Report
- An Extensive Epidural Abscess of Cervicothoracic Spine Resolved by a Combined Approach – a Case Report
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Neurosyphilis
- Surgical Treatment of a Tarsal Tunnel Syndrome
- Diagnosis and Treatment Options for Niemann-Pick Disease Type C
- Bilateral Phrenic Nerve Lesion Manifesting as an Orthopnea – Three Case Reports
