The Libechov Minipig as a Large Animal Model for Preclinical Research in Huntington’s disease – Thoughts and Perspectives
The Libechov Minipig as a Large Animal Model for Preclinical Research in Huntington’s disease – Thoughts and Perspectives
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors:
S. Schramke 1,2; R. Schubert 1; F. Frank 1,3; M. Wirsig 1; M. Fels 2; N. Kemper 2; V. Schuldenzucker 1; R. Reilmann 1,3,4
Authors‘ workplace:
George-Huntington-Institute, Muenster, Germany
1; Institute for Animal Hygiene, Animal Welfare and Farm Animal Behaviour, University of Veterinary Medicine Hannover, Germany
2; Institute for Clinical Radiology, University of Muenster, Germany
3; Department of Neurodegenerative Diseases and Hertie-Institute for Clinical Brain Research, University of Tuebingen, Germany
4
Published in:
Cesk Slov Neurol N 2015; 78/111(Supplementum 2): 55-60
doi:
https://doi.org/10.14735/amcsnn20152S55
Overview
Large animal models to explore the safety and tolerability of novel therapeutic approaches for Huntington’s disease (HD) are in exploration to achieve higher translational reliability in future studies. Recently, a Libechov minipig has been established as one new transgenic (Tg) large animal model for HD. We here discuss the advantages and limitations in using this model in HD with regards to breeding, housing, handling, and with respect to homology to humans and ethical considerations. A group of TgHD and wild type (WT) female minipigs (n = 36) was used to gain first evidence about abovementioned aspects. It is concluded that Libechov minipigs may fulfill an important role to bridge the gap between rodents and non‑human primates in the translation to humans.
Key words:
minipig – Huntington’s disease – animal model – phenotyping – magnetic resonance imaging – imaging – behavioral – preclinical research
A wide range of transgenic (Tg) and knock ‑ in animal models has been developed to explore the pathology, safety and efficacy of new therapeutic approaches for Huntington’s disease (HD) [1]. HD is an autosomal ‑ dominant neurodegenerative disorder with motor, cognitive and behavioral symptoms [2,3]. It is caused by a CAG triplet repeat expansion ≥ 36 in the huntington gene that leads to neuronal dysfunction and death in wide areas of the brain including the cerebral cortex, white matter and striatum, due to the misfolded mutant huntingtin (mHTT) protein [4 – 8]. Established animal models are e. g. nematodes, drosophila, mice, rats, sheep, monkeys and minipigs [9,10]. So far especially the rodent models contributed a major part of the preclinical research in HD [11 – 13]. In spite of numerous preclinical findings, none of the compounds proposed for disease modifying treatments of HD based on preclinical data has been success-fully translated into the clinic to date [14]. Large animal models have thus been proposed as a possible improvement for preclinical assessments with a higher probability for successful translation. One model recently established by the Research Center PIGMOD & Institute of Animal Physiology and Genetics, Academy of Sciences of the Czech Republic, Libechov, Czech Republic, is the TgHD Libechov minipig [15]. We decided to explore the value of the Libechov minipig as an animal model for HD with respect to breeding, housing and handling, and particularly with regards to aspects such as the similarity to humans and ethical considerations (Fig. 1). The TgHD Libechov minipig exhibited a stable transmission of the HD mutation across several generations. The Libechov minipig was created by using lentiviral transduction. It expresses an N‑terminal truncated form of human huntingtin with 124 CAG/ CAA repeats on chromosome 1. We here present and discuss arguments for and against applying this minipig as a large animal model for HD based on experience gained with the Libechov minipigs in a long term follow‑up study.

Tg and wild type (WT) Libechov minipigs (n = 36 total), bred in the Institute of Animal Physiology and Genetics of the Czech Academy of Science in Libechov, Czech Republic [15], were husbanded in Muenster. The animals arrived in the central animal facility of the University of Muenster, Germany, at an age of three months in six groups of six animals. The groups included female WT and TgHD animals. Each group was housed in a temperature and humidity controlled stable with a size of 2 m2 per animal. The stables are enriched with toys, litter and hay. A daily veterinary care was provided and weight was monitored weekly. Before the battery of phenotyping assessments started the animals initially passed a short phase of anti‑panic treatment. After successful habituation in the new environment the minipigs learnt to follow a target stick by using classical and operant conditioning to ensure a comfortable handling.
The battery of assessments developed and explored included several motor, cognitive and behavioral tests will be described elsewhere [16 – 20] The prerequisite for performing this battery of tests was the feasibility to successfully handle the animals. Further the minipigs underwent magnetic resonance imaging (MRI) scans that included multiple anatomical, diffusion ‑ weighted and spectroscopic sequences, which will also be described elsewhere in detail [21 – 24]. Precondition for performing MR Imaging during anesthesia was the feasibility to narcotize the animals for a longer period of time.
The experience with our 36 Libechov minipigs yielded a lot of arguments in favor of applying and further developing the minipig as a model for neurodegenerative disorders such as HD. However, we also discovered disadvantages of the model, which are discussed below.
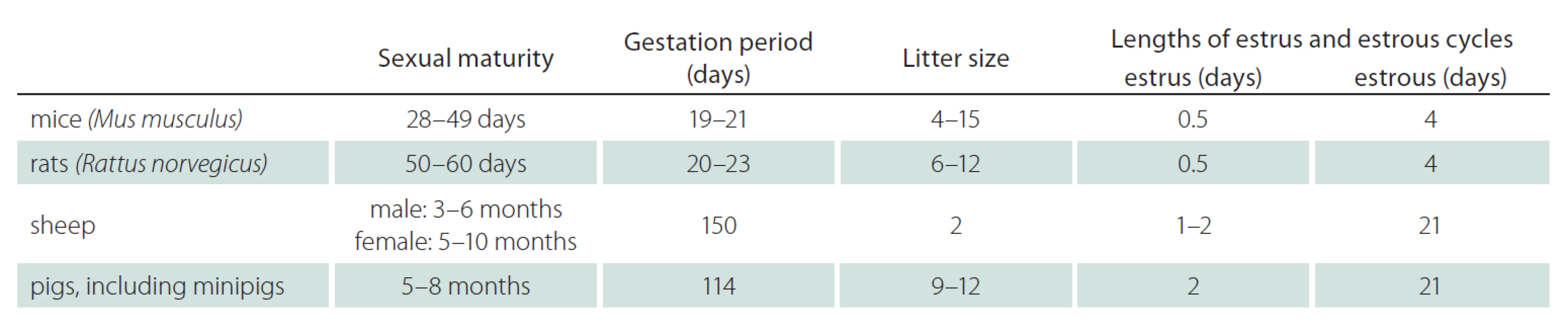
Tab. 1 shows the generation times of mice, rats, sheep and pigs in view of sexual maturity, gestation period, litter size and length of estrus and estrous cycles [9,25,26]. With the sexual maturity of 5 – 8 months (pigs) and 3 – 10 months (sheep), and the gestation period of 114 days (pigs) and 150 days (sheep) large animals show a longer generation time than rodents. The litter size and the lengths of estrus and estrous cycles support the use of rodents when large numbers of animals and short timelines are required. While sheep lamb only two offspring, pigs are able to breed 9 – 12 piglets. Thus, litter size favors pigs over sheep. In general, bigger litter sizes of genetically changed animals enable faster and more economic breeding with fewer founder ‑ animals.
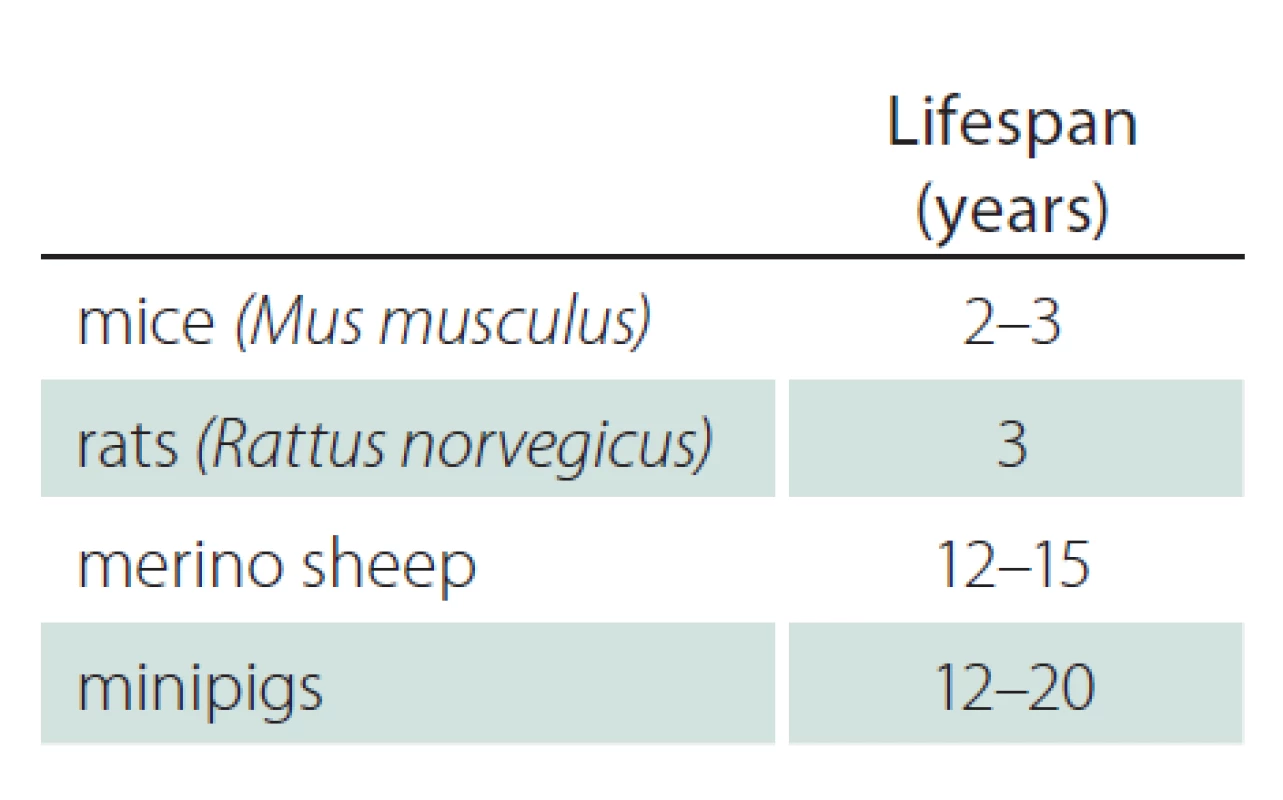
Tab. 2 shows the lifespan of mice, rats, sheep and minipigs [27 – 33]. While rodents are short‑lived, large animals have a lifespan up to 20 years. HD and other neurodegenerative disorders need many years to decades to manifest clinically. Therefore, a long lifespan may be advantageous to study the progression of disease with similar timelines than observed in human phenotype development.
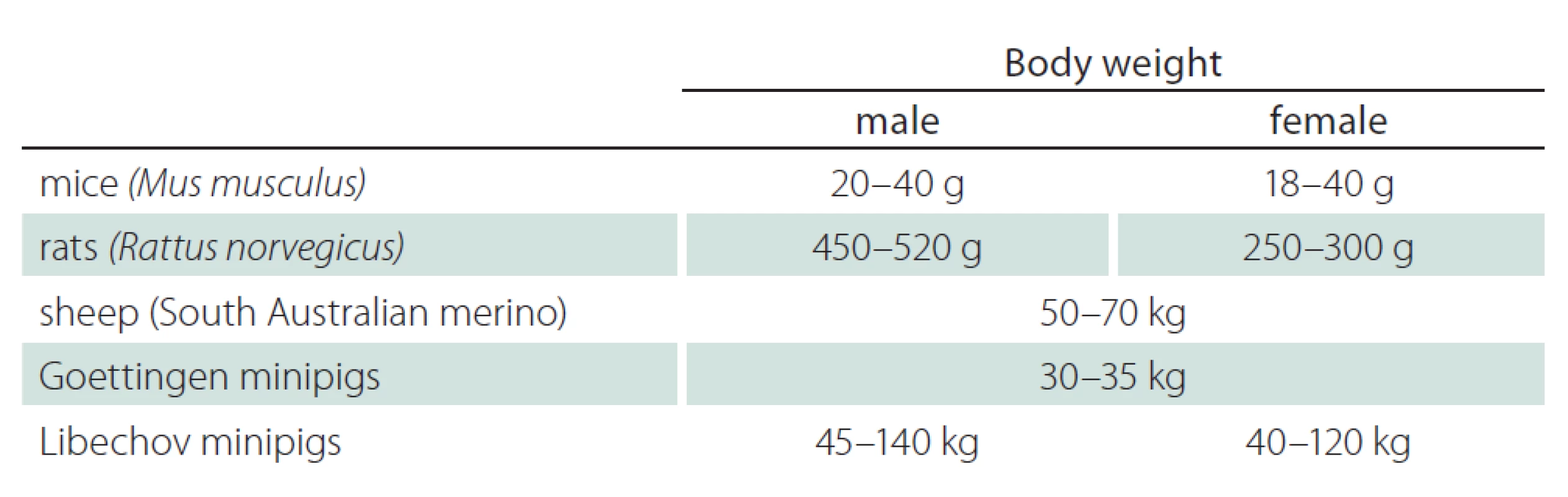
Tab. 3 shows the average body weight of mice, rats, sheep, Goettingen minipigs and Libechov minipigs [9,26,34,35]. Female Libechov minipigs have a mean body weight of around 75 kg at an age of 30 months (body weight at the age of 30 months across 32 female Libechov minipigs); thus, the weight of these animals is comparable to adult humans. However, considerable variability between body weights can be observed in both the Libechov minipigs and humans. Nevertheless, preclinical research in this model permits pharmacological studies with a biodistribution pattern that should allow reliable translation to humans. Food intake of the Libechov minipig must be controlled and these animals have to be fed restrictively to avoid unpredictable weight gains. To optimize husbandry minipigs should be separated while fed, because they develop a hierarchy that results in unequal access to food with the strongest minipigs receiving most. Large differences between individual body weights lead to problems with regards to handling. We did not observe relevant levels of aggression or problems with obedience. Handling and transporting the animals during narcosis, e. g. into the MRI scanner, is challenging, but feasible. Assistive devices or sufficient manpower are necessary to lift a pig with more than 75 kg body weight. In general, we found that minipigs are easy to sedate, intubate and anesthetize. Narcosis is stable and can be maintained for a long time and administered repeatedly.
The porcine digestive system is similar to the human. Pigs as opposed to sheep are omnivores and monogastric animals. Due to this, testing of oral therapeutics under similar conditions with respect to drug absorption is feasible. Additionally, pigs can be kept in back, prone, or lateral position permitting to perform diverse manipulations during narcosis. In general, minipigs are well‑studied and established. They play an important role as model for preclinical research in many different diseases, e. g. in neurodegenerative, reproductive, cardiovascular and metabolic diseases, and in surgery.
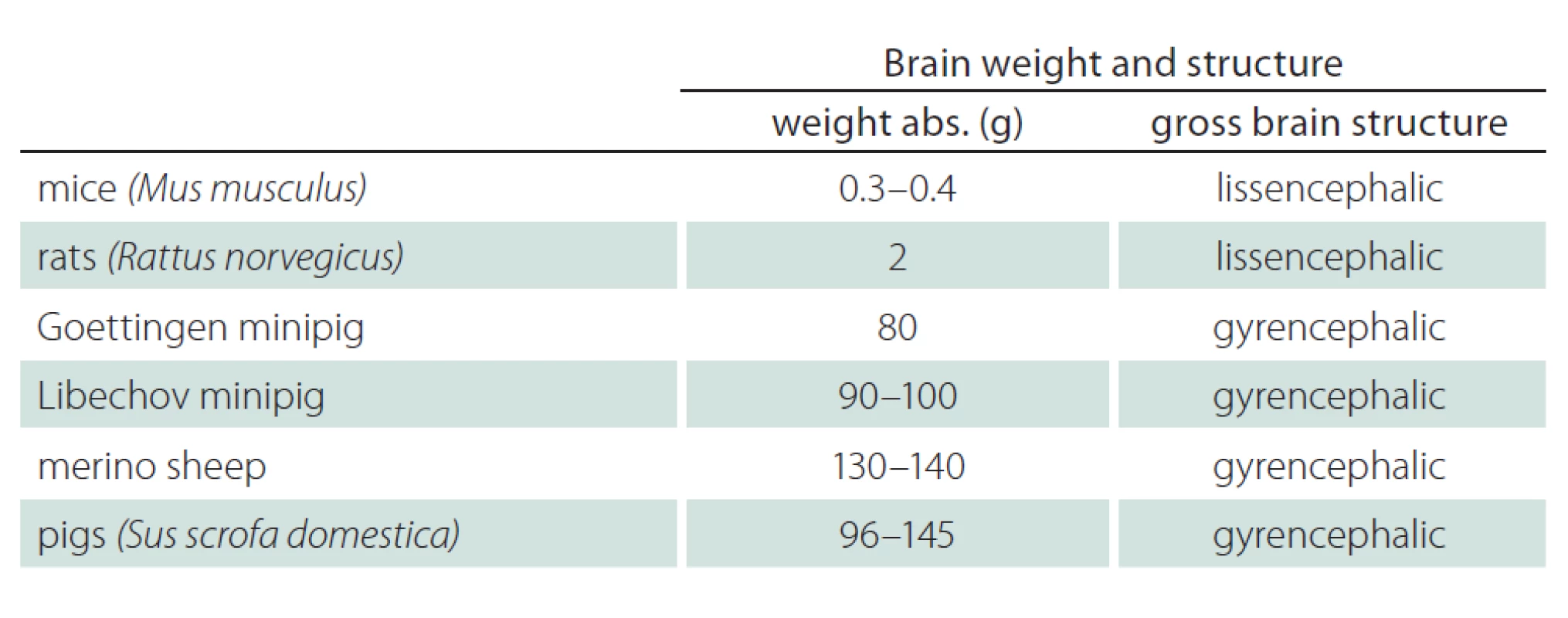
Tab. 4 and Fig. 2 show the brain volume and characteristics of the gross brain structure of mice, rats, minipigs, pigs, and sheep [9,36 – 39]. Brain volume and brain structure should be important aspects when selecting suitable animals for preclinical research in neurodegenerative disorders. Pigs, Libechov minipigs and sheep have a brain volume of 96 – 145 g, 90 – 100 g and 130 – 140 g, respectively. The brain size of human is approximately 1,300 – 1,400 g. Thus there still is a considerable difference between the brain size of large animals and humans. Nevertheless, the sheep’s, minipig’s and pig’s brain size and structure offer advantages compared to rodents as demonstrated by MRI and positron emission tomography (PET) applications in vivo. Large animals have a gyrencephalic brain similar to humans while rodents have a lissencephalic brain. The brain of minipigs is also similar to humans with regards to the blood supply and to immune response characteristics [32]. However, a serious disadvantage of minipigs compared to e. g. sheep are the large paranasal sinuses. This makes brain implants a difficult challenge in minipigs, while implants in sheep brains are possible due to a different anatomy [9].

Six female groups with six WT and TgHD animals were housed in the central animal facility of the University of Muenster, Germany. Each group was housed in a stable with 2 m2 per animal. The stables were temperature and humidity controlled. Pigs are not able to sweat. They increase their temperature by using other pigs or litter as heat source. To reduce temperature they lie alone and decrease their food intake. Adult pigs have a temperature optimum of 15 – 20 °C. In Muenster, all animals had the possibility to use toys like balls, chains and sisal 24 hours a day. Minipigs are curious animals and ready to explore new items. Balls and teeth rings were fixed with chains. Every month the toys were rotated between groups to preserve their interest. In addition, stables were enriched with chains, sisal, litter and hay (Fig. 3).

The minipigs exhibited high levels of motivation to cooperate with the experimenters. The animals were easy to handle and easily pleased for several years. However, work with minipigs requires more manpower and more space compared to work with rodents.
Female minipigs and castrated males live in groups. Social hierarchy between minipigs in one group is strong and persists. Because of this constant hierarchy behavioral changes with impact on social interaction should be readily detectable. Thus we expect that the complex social structure is another feature of the minipigs that increases the comparability to human HD.
Ethical consideration plays an increasing and important role in animal research. The principles of the 3Rs were established is an effort to Replace, Reduce and Refine animal research. William Russell and Rex Burch developed this strategy in 1959 in “The Principles of Humane Experimental Technique” [40].
The idea is to replace animal models with in vitro alternatives wherever possible, to reduce the number of animals to a minimum when animal models are indispensable and to refine the experimental procedures to minimize pain and distress (Fig. 4).

Unfortunately it is not possible to replace animals with alternative methods in several fields including preclinical research in HD, where animal models are still indispensable. Nevertheless, the overall effort to reducing the number of animals applied in research to a minimum should be in our focus. Also undisputed is that every animal we use – no matter what species – should be enriched during the whole lifespan: breeding, transporting, housing, handling, health maintenance, methods of euthanasia or detailed consideration whether there is the opportunity to rehome or to retire the animals should be considered wherever possible.
The social tolerance for animal research decreases with an increasing similarity of the species applied to humans. While animal research in rodents is mostly accepted, research in non‑human primates causes major problems with animal rights activists. Farm animals such as sheep and pigs are more socially accepted as research models than primates or animals that are frequently kept as pets (e. g., dogs).

While the need for large animal models for HD may be undisputed, the development of these models is work in progress. A considerable advantage of large animal models such as minipigs is the feasibility to perform assessments in vivo such as MRI, PET, CSF (Collecting Cerebrospinal Fluid), blood collection, and stereotactically ‑ guided delivery of drugs into the brain [9,41]. Another advantage of large animals such as minipigs is the high genetic homology to humans, in general and with respect to the htt gene. The porcine htt gene, for instance, has higher genetic homology to humans (96%) [15] than the htt gene in rodents, e. g. mice with 91% [42].
However, the Libechov minipig currently used has limitations due to the genetic construct used, which only expresses a fragment of the N‑terminal part of the huntington gene. In addition, the model uses a CAG/ CAA repeat while the human huntington gene has a pure CAG repeat. The fact that only one transgene is inserted may be advantageous, however, it must be kept in mind that the huntingtin fragment is expressed with the background of two porcine huntington genes. Therefore a desirable next step would be the development of a humanized knock ‑ in minipig model of HD.
Another serious limitation is the lack of reliable data on the time and course of phenotype development in the TgHD Libechov minipig model. However, characterization of the model with behavioral tests [16 – 20] and a range of imaging measures [21 – 24] translated from human studies such as TRACK ‑ HD is ongoing and results will be available soon. Once the timeline and magnitude of phenotype measures is known, studies may expand from safety and tolerability or biomarker assessments, which are available today, to symptomatic and disease modifying trials using clinical endpoints.
In spite of the availability of several HD animal models, the pathomechanisms of HD are still not fully understood. Likely, the animal models established to date will not be able to answer relevant outstanding questions. All of them exhibit advantages and disadvantages (Fig. 5). Large animal models, such as the TgHD minipig discussed here, offer the unique opportunity to expand our knowledge. They may serve as a valuable compromise between scientific needs and environmental requirements. Thus they could occupy a central position between rodents and non‑human primates, close the gap between preclinical research in rodents and clinical research in humans and contribute to higher translational reliability and sensitivity in HD and beyond. The use of TgHD minipigs in preclinical studies is feasible, and a further development of this model both in terms of assessments and advances in genetic constructs is warranted.
The “TRACK ‑ TgHD Minipig” study was funded by the CHDI foundation (www.chdifoundation.org). We are indebted to all patients and their families who support the work of the George ‑ Huntington ‑ Institute and thus provided additional funding for this work. We thank Jan Motlik, Monika Baxa and Stefan Juhas from the Research Center PIGMOD & Institute of Animal Physiology and Genetics, Academy of Sciences of the Czech Republic, Libechov, Czech Republic, for support and for breeding and providing the WT and TgHD minipigs. We are also grateful for the generous support of the Faculty of Medicine of the University of Muenster, particularly the Central Animal Facility represented by Stefan Schlatt, Jens Ehmcke, Sandra Stoeppeler and the whole team at the facility. Valuable administrative advice has been given by Martin Luecke. Some elements of this publication are part of the unpublished dissertations of Sarah Schramke, Verena Schuldenzucker, Maike Wirsig and Eva Hoelzner.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Accepted for review: 24. 9. 2015
Accepted for print: 19. 10. 2015
Sarah Schramke
George-Huntington-Institute
Technology Park Muenster
Johann-Krane Weg 27
481 49 Muenster
Germany
e-mail: sarah.schramke@ghi-muenster.de
Sources
1. Chang KH, Wu YR, Chen YC, Chen CM. Plasma inflammatory biomarkers for Huntington‘s disease patients and mouse model. Brain Behav Immun 2015; 44 : 121 – 127. doi: 10.1016/ j.bbi.2014.09.011.
2. Ross CA, Pantelyat A, Kogan J, Brandt J. Determinants of functional disability in Huntington‘s disease: role of cognitive and motor dysfunction. Mov Disord 2014; 29 : 1351 – 1358. doi: 10.1002/ mds.26012.
3. Walker FO. Huntington‘s disease. Lancet 2007; 369(9557): 218 – 228.
4. Aylward EH, Harrington DL, Mills JA, Nopoulos PC, Ross CA, Long JD et al. Regional atrophy associated with cognitive and motor function in prodromal Huntington‘s disease. J Huntingtons Dis 2013; 2(4): 477 – 489. doi: 10.3233/ JHD ‑ 130076.
5. MacDonald ME, Ambrose CM, Duyao MP, Myers RH, Lin C, Srinidhi L et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington‘s disease chromosomes. Cell 1993; 72(6): 971 – 983.
6. Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D et al. Biological and clinical manifestations of Huntington‘s disease in the longitudinal TRACK ‑ HD study: cross ‑ sectional analysis of baseline data. Lancet Neurol 2009; 8(9): 791 – 801. doi: 10.1016/ S1474 ‑ 4422(09)70170 ‑ X.
7. Tabrizi SJ, Scahill RI, Owen G, Durr A, Leavitt BR, Roos RAet al. Predictors of phenotypic progression and disease onset in premanifest and early‑stage Huntington‘s disease in the TRACK ‑ HD study: analysis of 36 - month observational data. Lancet Neurol 2013; 12(7): 637 – 649. doi: 10.1016/ S1474 ‑ 4422(13)70088 ‑ 7.
8. The Huntington‘s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington‘s disease chromosomes. Cell 1993; 72(6): 971 – 983.
9. Morton AJ, Howland DS. Large genetic animal models of Huntington‘s disease. J Huntingtons Dis 2013; 2(1): 3 – 19. doi: 10.3233/ JHD ‑ 130050.
10. Pouladi MA, Morton AJ, Hayden MR. Choosing an animal model for the study of Huntington‘s disease. Nat Rev Neurosci 2013; 14(10): 708 – 721. doi: 10.1038/ nrn3570.
11. Crook ZR, Housman D. Huntington‘s disease: can mice lead the way to treatment? Neuron 2011; 69(3): 423 – 435. doi: 10.1016/ j.neuron.2010.12.035.
12. Kim J, Bordiuk OL, Ferrante RJ. Experimental models of HD and reflection on therapeutic strategies. Int Rev Neurobiol 2011; 98 : 419 – 481. doi: 10.1016/ B978 ‑ 0 ‑ 12 ‑ 381328 ‑ 2.00016 ‑ X.
13. William YX, Gray M. Mouse Models for Validating Preclinical Candidates for Huntington‘s Disease. In Lo DC, Hughes RE (eds). Neurobiology of Huntington‘s disease: Applications to Drug Discovery. CRC Press: Boca Raton 2011.
14. Venuto CS, McGarry A, Ma Q, Kieburtz K. Pharmacologic approaches to the treatment of Huntington‘s disease. Mov Disord 2012; 27(1): 31 – 41. doi: 10.1002/ mds.23953.
15. Baxa M, Hruska ‑ Plochan M, Juhas S, Vodicka P, Pavlok A,Juhasova J et al. A transgenic minipig model of Huntington‘s disease. J Huntingtons Dis 2013; 2(1): 47 – 68. doi: 10.3233/ JHD ‑ 130001.
16. Ott S, Schramke S, Schuldenzucker V, Wirsig M, Schubert R, Frank F et al. Track tgHD Minipig – Assessing Resource Holding Potential Behaviour as part of a Battery for Phenotyping tgHD Minipigs. J Neurol Neurosurg Psychiatry 2014; 85: A31.
17. Schramke S, Schuldenzucker V, Schubert R, Frank F, Wirsig M, Ott S et al. Behavioral phenotyping of minipigs transgenic for the Huntington gene. Unpublished.
18. Schramke S, Schuldenzucker V, Ott S, Wirsig M, Frank F,Schubert R et al. Track TgHD minipigs – Discrimination Test as a part of an assessment battery for TgHD minipigs. J Neurol Neurosurg Psychiatry 2014; 85: A31.
19. Schuldenzucker V, Schramke S, Wirsig M, Ott S, Schubert R, Frank F et al. Track TgHD minipig – assessment of motor function. J Neurol Neurosurg Psychiatry 2014; 85: A30.
20. Wirsig M, Schuldenzucker V, Schramke S, Frank F, Schubert R, Ott S et al. Track TgHD minipig – Startbox back and Forth Test. J Neurol Neurosurg Psychiatry 2014; 85: A30 – A31.
21. Frank F, Nagelmann N, Liebsch L, Schubert R, Wirsig M, Schramke S al. Striatal Magnetic Resonance Spectroscopy of Transgenic HD Minipigs. J Neurol Neurosurg Psychiatry 2014; 85: A29 – A30.
22. Nagelmann N, Frank F, Liebsch L, Schubert R, Wirsig M,Schramke S et al. Volumetry of Nucleus Caudatus, Lateral Ventricles and Cerebrum of Founder and Second Generation Libechov Transgenic HD Minipigs. J Neurol Neurosurg Psychiatry 2014; 85: A29.
23. Schubert R, Frank F, Nagelmann N, Liebsch L, Schuldenzucker V, Schramke S et al. Neuroimaging of a minipig model of Huntington‘s disease: feasibility of volumetric, diffusion ‑ weighted and spectroscopic assessments. Unpublished.
24. Schubert R, Frank F, Nagelmann N, Schramke S, Schuldenzucker V et al. MR‑based Stereotaxic Standard Brain Atlas Of The Libechov Minipig. J Neurol Neurosurg Psychiatry 2014; 85: A29.
25. Nickel R, Schummer A, Frewein J, Seiferle E. Eingeweide. Lehrbuch der Anatomie der Haustiere 2. Stuttgart: Parey 2004.
26. Wolfensohn S, Lloyd M. Handbook of Laboratory Animal Management and Welfare. Wiley: Blackwell Publishing 2013.
27. Boujard D, Anselme B, Cullin C, Raguénès ‑ Nicol C, Lechowski S. Zell ‑ und Molekularbiologie im Überblick. Berlin: Springer Heidelberg 2014.
28. Busch B, Blaha T. Der Tierheim ‑ Leitfaden: management und artgemäße Haltung. Verlag: Schattauer 2013.
29. Gabrisch K, Fehr PD, Sassenburg L, Zwart PD. Krankheiten der Heimtiere. Sassenbrug: Schlütersche Verlagsgesellschaft mbH & Company KG 2014.
30. Hagemann E, Schmidt GE. Ratte und Maus: versuchstiere in der Forschung. Berlin: W. de Gruyter 1960.
31. McAnulty PA, Dayan AD, Ganderup NC, Hastings KL. The Minipig in Biomedical Research. Boca Raton: CRC Press 2011.
32. Vodicka P, Smetana K jr, Dvorankova BF, Emerick TF, Xu YZ, Ourednik J et al. The miniature pig as an animal model in biomedical research. Ann N Y Acad Sci 2005; 1049 : 161 – 171.
33. Weiss J, Becker K, Bernsmann E, Chourbaji S, Dietrich H.Versuchstierkunde: Tierpflege in Forschung und Klinik. Berlin: Enke 2014.
34. Bollen PF, Ellegaard L. The Gottingen minipig in pharmacology and toxicology. Pharmacol Toxicol 1997; 80 (Suppl 2): 3 – 4.
35. Bollen PJ, Hansen AK, Alstrup AK. The Laboratory Swine. 2nd ed. Washington: CRC Press 2010.
36. Böhme G. Lehrbuch der Anatomie der Haustiere Band 4: Nervensystem, Sinnesorgane, Endokrine Drüsen. Berlin: Parey 2004.
37. Jelsing J, Gundersen H jr, Nielsen R, Hemmingsen R, Pakkenberg B. The postnatal development of cerebellar Purkinje cells in the Goettingen minipig estimated with a new stereological sampling technique – the vertical bar fractionator. J Anatomy 2006; 209 : 321 – 331.
38. Jerison H. Evolution of The Brain and Intelligence. New York: Elsevier Science 2012.
39. Roth G. Das Gehirn und seine Wirklichkeit: kognitive Neurobiologie und ihre philosophischen Konsequenzen. Berlin: Suhrkamp 1997.
40. Flecknell P. Replacement, reduction and refinement. Altex 2002; 19(2): 73 – 78.
41. Bjarkam CR, Cancian G, Glud AN, Ettrup KS, Jorgensen RL, Sorensen JC. MRI ‑ guided stereotaxic targeting in pigs based on a stereotaxic localizer box fitted with an isocentric frame and use of SurgiPlan computer ‑ planning software. J Neurosci Methods 2009; 183(2): 119 – 126. doi: 10.1016/ j.jneumeth.2009.06.019.
42. Kosinski C, Cha JH, Young AB, Schwarz M. Chorea Huntington Tiermodelle eröffnen neue Hypothesen zu Pathophysiologie und Therapie. Nervenarzt 1999; 70(10): 878 – 888.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2015 Issue Supplementum 2
-
All articles in this issue
- Registry of authors
- 31P MR Spectroscopy of the Testes and Immunohistochemical Analysis of Sperm of Transgenic Boars Carried N‑terminal Part of Human Mutated Huntingtin
- Acyl‑ CoA Binding Domain Containing 3 (ACBD3) Protein in Huntington’s Disease Human Skin Fibroblasts
- Telemetry Physical Activity Monitoring in Minipig’s Model of Huntington’s Disease
- The Effect of Melatonin on Proliferation of Primary Porcine Cells Expressing Mutated Huntingtin
- Buccal Epithelial Cells as Potential Non‑ invasive Materials for the Monitoring of Mitochondrial Disturbances to Track Huntington‘s Disease Progression – a Pilot Study
- The Libechov Minipig as a Large Animal Model for Preclinical Research in Huntington’s disease – Thoughts and Perspectives
- Grunting in a Genetically Modified Minipig Animal Model for Huntington’s Disease – Pilot Experiments
- Different Forms of Huntingtin in the Most Affected Organs; Brain and Testes of Transgenic Minipigs
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- The Libechov Minipig as a Large Animal Model for Preclinical Research in Huntington’s disease – Thoughts and Perspectives
- Buccal Epithelial Cells as Potential Non‑ invasive Materials for the Monitoring of Mitochondrial Disturbances to Track Huntington‘s Disease Progression – a Pilot Study
- Telemetry Physical Activity Monitoring in Minipig’s Model of Huntington’s Disease
- Grunting in a Genetically Modified Minipig Animal Model for Huntington’s Disease – Pilot Experiments
