Anterior Ischemic Optic Neuropathy and Branch Retinal Artery Occlusion after Transcatheter Closure of Foramen Ovale – a Case Report
Přední ischemická optická neuropatie a okluze větve retinální tepny po transkatétrové okluzi foramen ovale – kazuistika
Tato kazuistika dokumentuje pozdní oftalmologické komplikace po transkatétrové okluzi foramen ovale patens u mladé pacientky. Žena (39 let) podstoupila transkatétrovou okluzi foramen ovale patens s následným odloženým (po čtyřech letech) rozvojem přední ischemické optické neuropatie na levém oku, zřejmě v důsledku paradoxní embolizace. Její stav byl dále komplikován okluzí větve retinální tepny na stejném oku. Objektivní nález a subjektivní problémy se zlepšily po provedení dekomprese pochvy zrakového nervu a po sérii vazodilatačních infuzí. Lékaři si musí být vědomi možnosti současného výskytu přední ischemické optické neuropatie a okluze větve retinální tepny u mladých pacientů, a to i několik let po okluzi foramen ovale patens, a možné účinnosti intenzivní vazodilatační terapie a časné dekomprese pochvy zrakového nervu.
Klíčová slova:
foramen ovale patent – transkatétrová okluze – komplikace – přední ischemická optická neuropatie – okluze větve retinální tepny
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors:
A. Stepanov 1; J. Studnicka 1; L. Hejsek 1; N. Jirásková 1; R. Herzig 2; P. Rozsíval 1; M. Valis 2
Authors‘ workplace:
Department of Ophthalmology, Faculty of Medicine, Charles University and University Hospital Hradec Kralove
1; Department of Neurology, Comprehensive Stroke Center, Faculty of Medicine, Charles University and University Hospital Hradec Kralove
2
Published in:
Cesk Slov Neurol N 2016; 79/112(4): 458-462
Category:
Case Report
doi:
https://doi.org/10.14735/amcsnn2016458
Overview
This case report illustrates delayed ophthalmologic complications after transcatheter closure of patent foramen ovale in a young patient. A 39-year-old woman underwent transcatheter closure of patent foramen ovale with subsequent delayed (after four years) development of the anterior ischemic optic neuropathy in the left eye, possibly due to paradoxical embolization. The patient’s condition was further complicated by branch retinal artery occlusion in the same eye. The objective finding and subjective problems improved following optic nerve sheath decompression and a series of vasodilator infusions. Physicians should be aware of possible simultaneous occurrence of anterior ischemic optic neuropathy and retinal artery occlusion in young patients, even several years after patent foramen ovale closure, and of possible effectiveness of intensive vasodilation therapy and early decompression of the optic nerve sheaths.
Key words:
patent foramen ovale – transcatheter closure – complications – anterior ischemic optic neuropathy – branch retinal artery occlusion
Introduction
Anterior ischemic optic neuropathy (AION) develops due to hypoperfusion of the optic nerve, supplied by posterior ciliary arteries, and usually occurs in patients over the age of 50 [1]. Annual incidence of the non-arteritic form of the AION is estimated to be 10.3 per 100,000 inhabitants older than 50 years and 0.54 per 100,000 inhabitants in all age groups [2]. Patients younger than 50 years represent 10.5–12.5% of all AION cases [3]. It is assumed that general vascular diseases, e. g. diabetes mellitus, decompensated arterial hypertension, decompensated renal insufficiency, arterial hypotension or anemia represent predisposing factors for the AION in patients younger than 50 years. No clinically effective treatment exists, largely because little is known about its pathophysiology [4].
The disease is manifested by sudden impairment of visual functions, relative afferent pupillary defect and edema of the optic disc. Beside the local (anatomical and system) hemodynamic factors, thrombophilic factors, such as antiphospholipid antibodies, protein C and S deficiency, antithrombin deficiency, tissue plasminogen activator deficiency, hyperhomocysteinemia, heterozygous mutation of factor V (Leiden) and MTHFR mutation are also engaged in the AION pathogenesis.
AION may also develop due to paradoxical embolization, for example on the basis of patent foramen ovale (PFO). PFO is a relatively common variant of physiological condition and occurs in 20–30% of general population subjects. Its potential risk is represented by possible passage of a thrombus from venous circulation to the left atrium and to system circulation. Multiple embolizations involving small thrombi several millimeters in size are more common than an embolization of a large thrombus. These thrombi undergo spontaneous lysis in the pulmonary circulation without major clinical sequelae, however, embolization may have serious consequences in the systemic arterial circulation, e. g. cerebral.
Central retinal artery supplying the retina is a terminal branch of the ophthalmic artery, the first intracranial branch of the internal carotid artery. Acute retinal ischemia and permanent functional impairment in the corresponding location develop following occlusion of this artery by a thrombus or embolus. The incidence of retinal artery occlusion (RAO) or its branches in patients younger than 40 years is estimated to be less than 1 per 50,000 [5]. The most common causes of RAO include atherosclerosis, embolism, arterial spasm, trauma, coagulopathy, thrombophlebitis, cavernous sinus thrombosis, polyarteritis nodosa and giant cell temporal arteritis. RAO is only rarely associated with an optic nerve affection, including the AION [6].
Case report
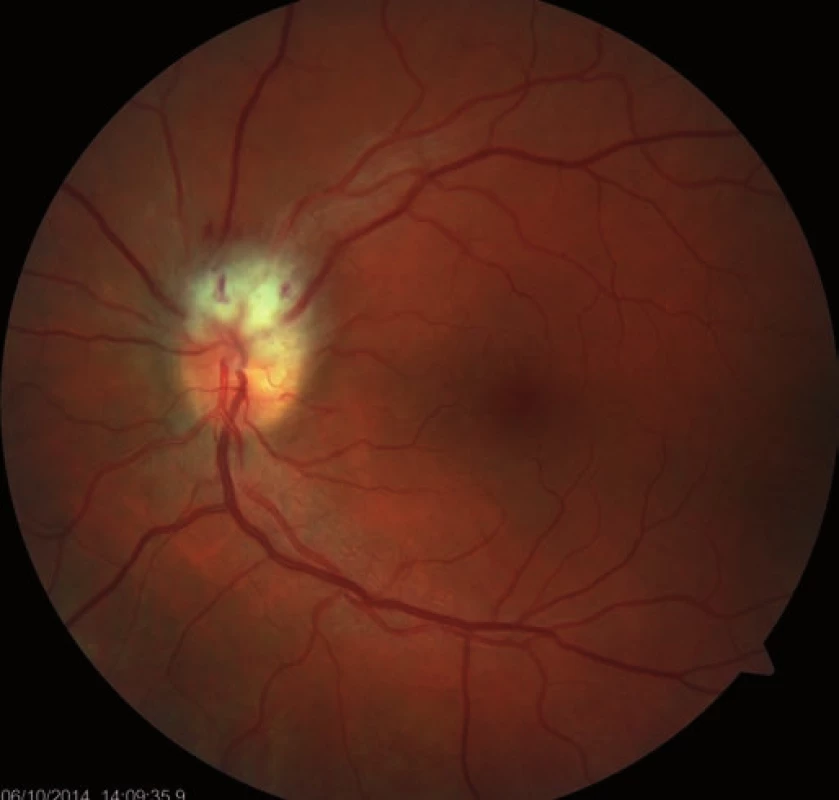
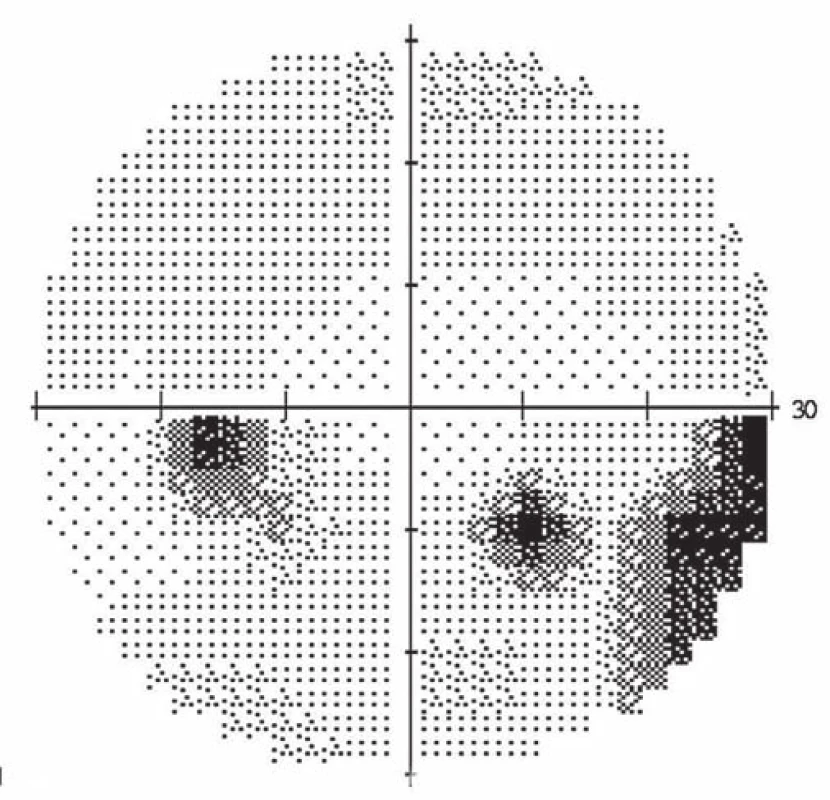
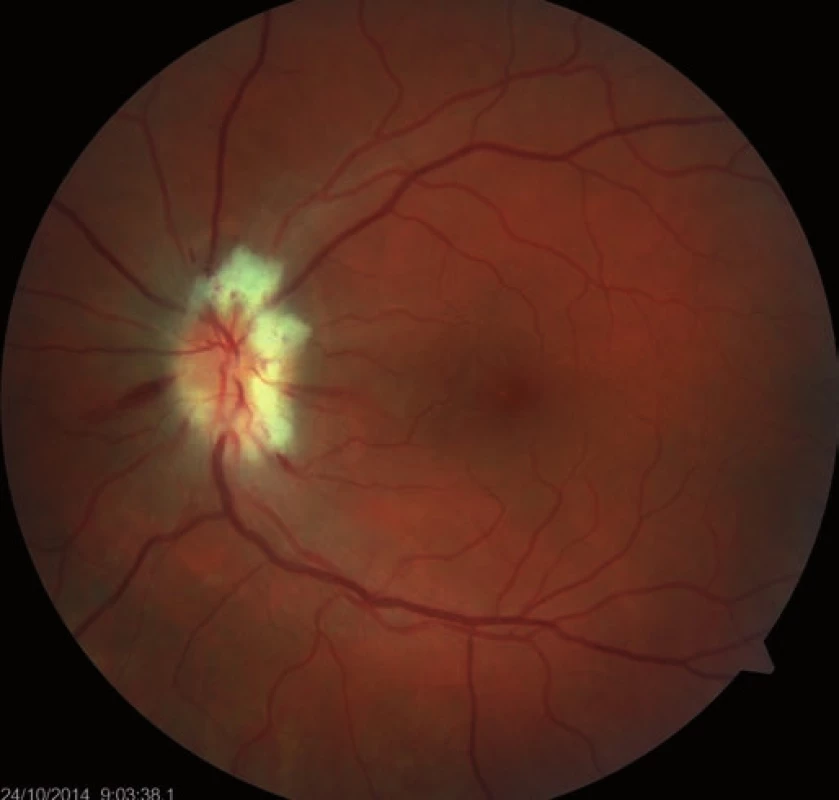
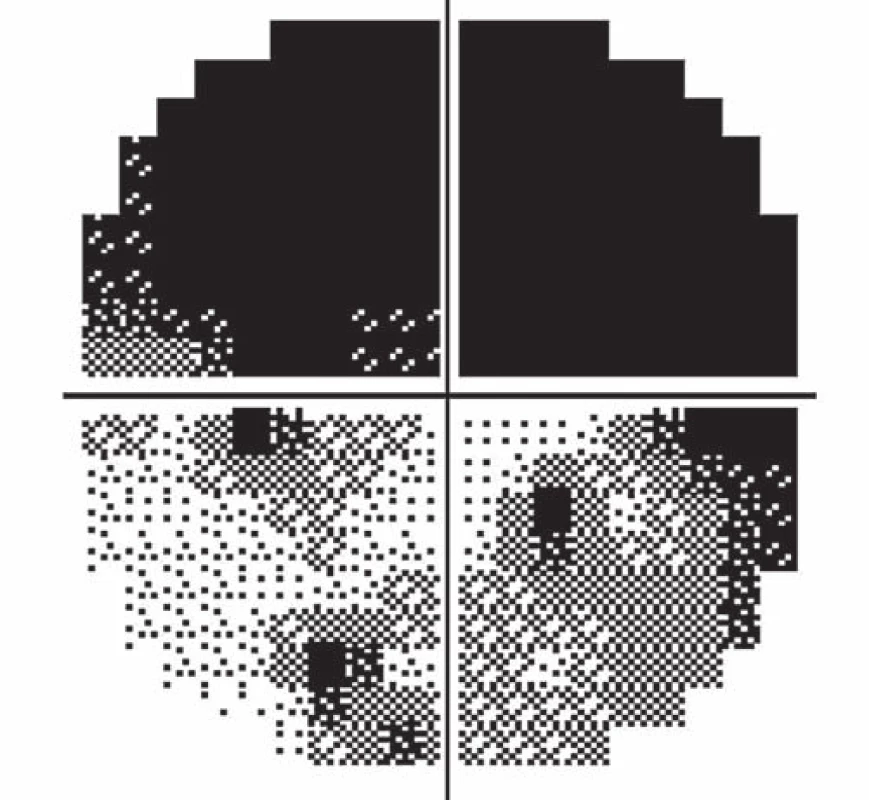
A 39-year-old woman had a history of bronchial asthma (with systemic treatment) and underwent transcatheter closure of the PFO using the Amplatzer® Septal Occluder (AGA Medical Corporation, Golden Valley, MN, USA) in 2010 because of post-ischemic changes randomly found on the brain magnetic resonance imaging (MRI) performed in 2008. Her medication also included acetylsalicylic acid (ASA) 100 mg a day orally. In November 2014, a blurred vision in the left eye developed. The patient signed an informed consent form for the suitable and available diagnostics and treatment. Visual acuity was 6/7.5 (20/25) and 6/6 (20/20), in the left and the right eye, respectively. The finding in the anterior eye segment bilaterally corresponded to the age of the patient. During fundoscopy of the left eye, a pale ischemic edema of the optic nerve with fuzzy edges in the upper half of the disc with several attached cotton wool-like foci and hemorrhages were found. The lower half of the optic nerve disc was delimited and other findings in the retina did not indicate any pathological changes (Fig. 1). The finding at the back of the right eye was adequate to the patient’s age. Visual field examination (perimeter Zeiss Humphrey, test T 30-2) of the left eye revealed lower altitudinal scotoma (Fig. 2). Lumbar puncture was performed with normal findings, including tensiometry. Thrombophilia was excluded by hematological examination and, the pathological finding in extracranial brain arteries was excluded using neurosonological examination. Transthoracic echocardiography confirmed correct function of the occlusion device in the PFO and no thrombus was found intracardially. MRI examination revealed postischemic changes in periventricular location and in the left cerebellar hemisphere. AION in the left eye was diagnosed. Antiplatelet therapy with ASA was replaced with clopidogrel 75 mg a day orally.
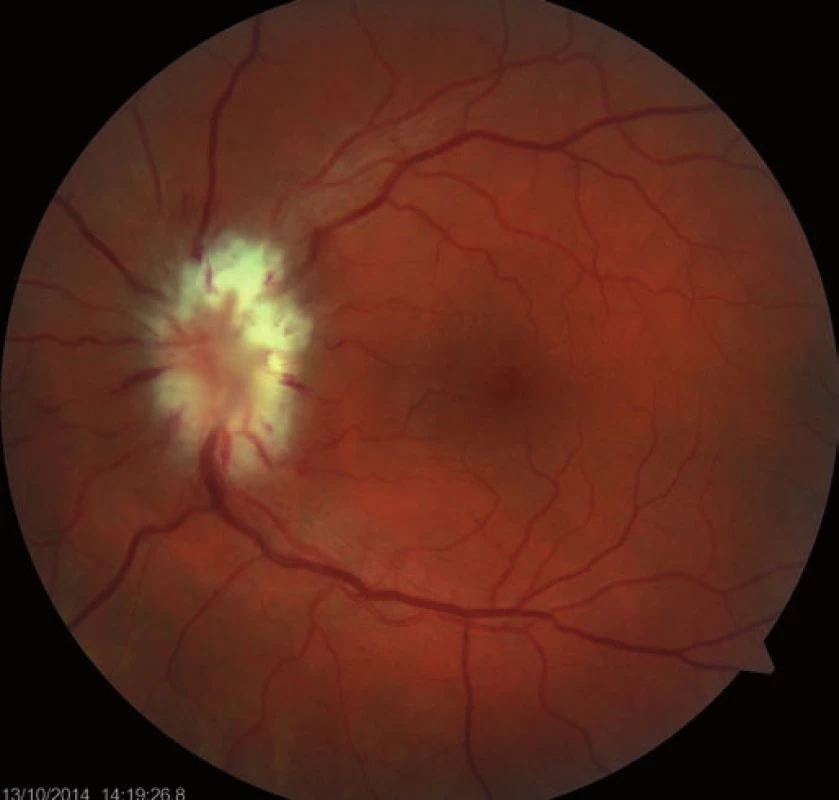
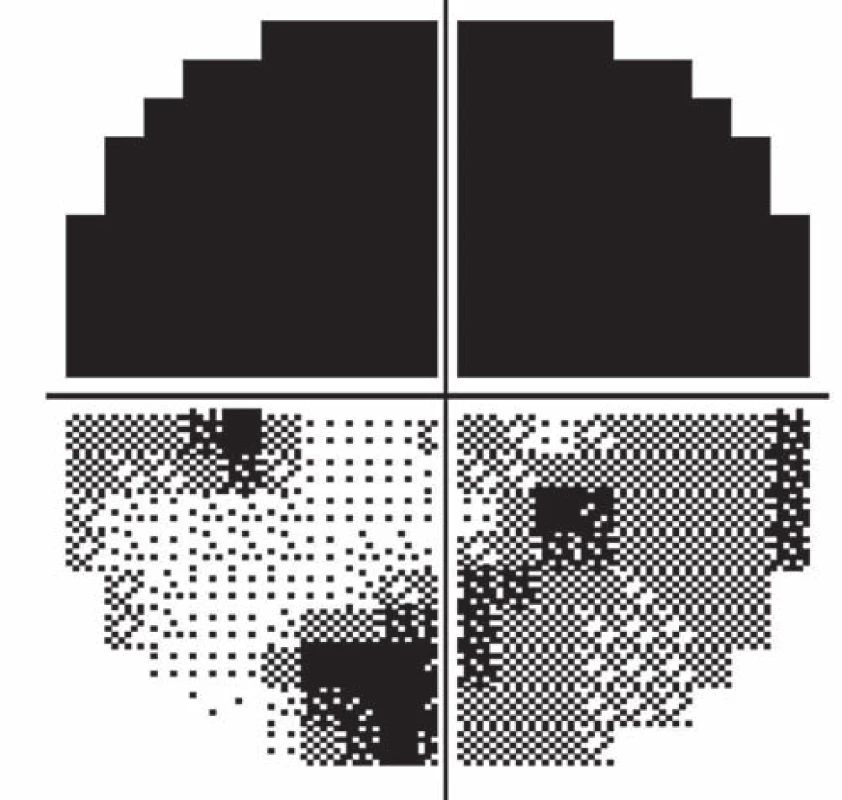
Follow-up ophthalmologic examination performed seven days later revealed significant worsening of the perimeter as well as the fundoscopic finding in the left eye. Optic nerve disc edema increased (Fig. 3) and the perimeter showed absolute scotoma in the upper half and absolute and relative defects with a preserved center in the lower part of the visual field (Fig. 4). Visual acuity was 6/ 10 (20/ 32). Decompression of the optic nerve sheaths in the left eye was performed to relief the progressing edema. Both subjective and objective improvement in the left eye was observed three days after the surgery, with gradual regression of the optic nerve disc edema (Fig. 5). Perimeter showed insignificant improvement of defects (Fig. 6) and visual acuity improved to 6/7.5 (20/25).

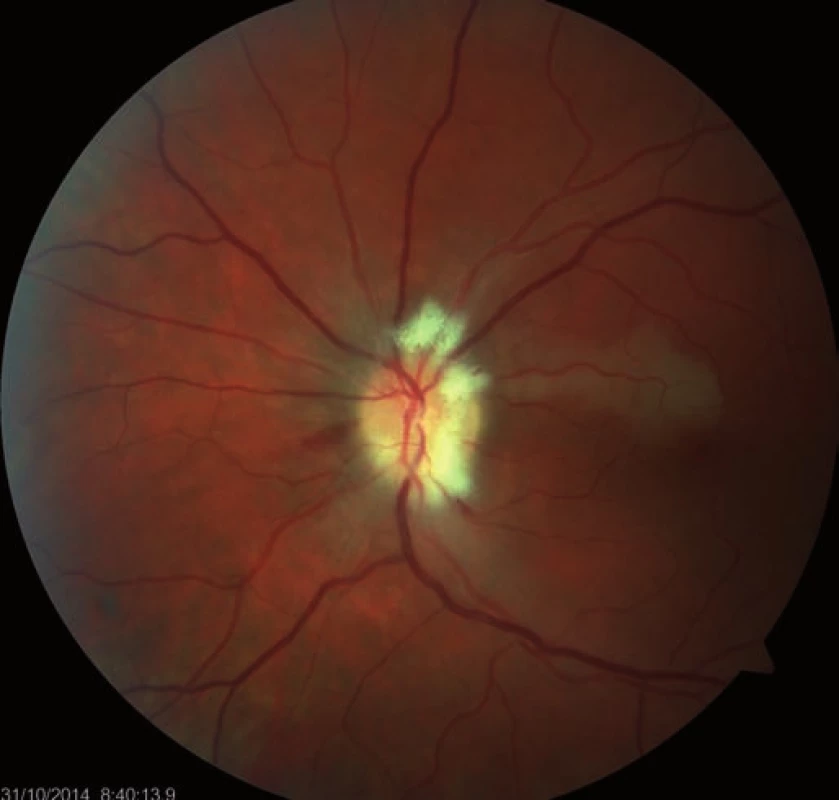
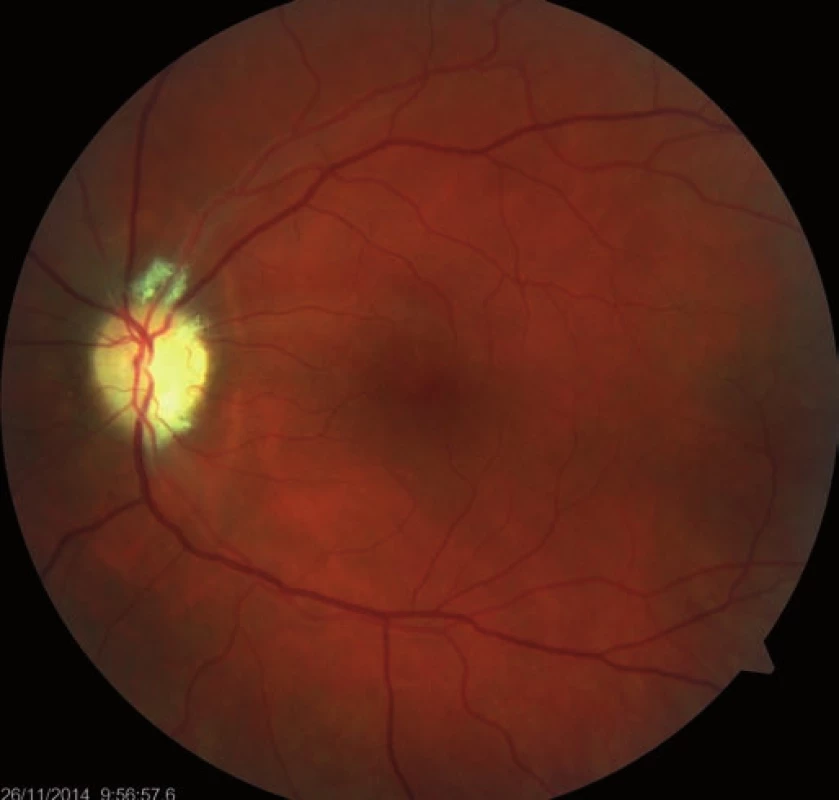
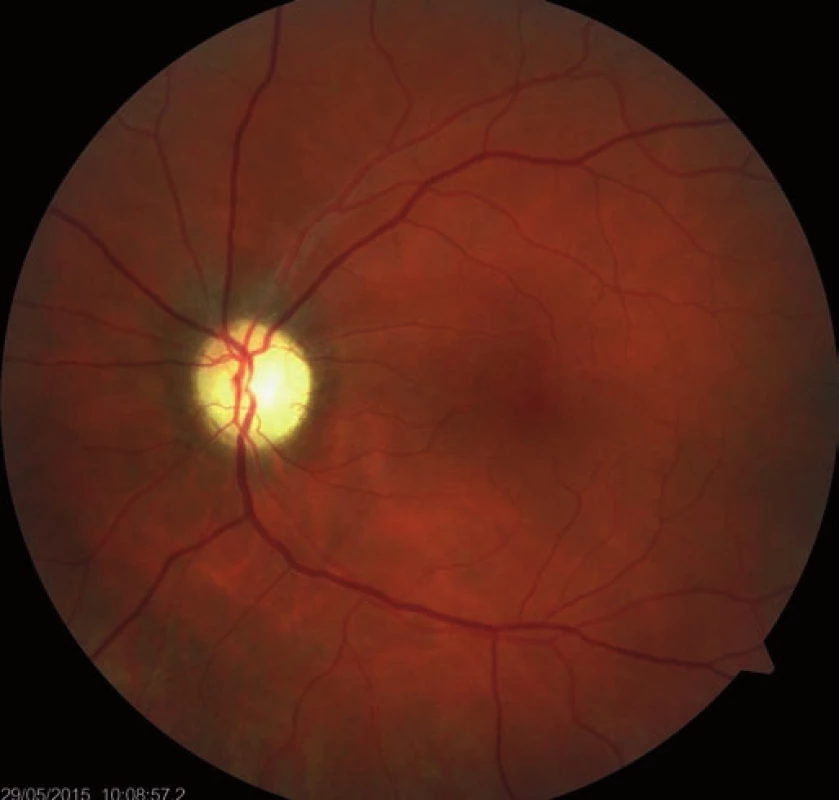
Fourteen days following discharge from the hospital, the patient reported subjective worsening of visual acuity in the left eye, which was 6/ 30 (20/ 100). At the back of the left eye, regression of the optic nerve disc edema and recurrence of pale edema along the upper macular branch of the central retinal artery were observed (Fig. 7). Fluorescence angiography demonstrated delayed filling of vascular circulation proximal to the edema and presence of cilioretinal artery. Optic coherence tomography of the central region of the left eye showed hyperreflexive neuroretinal structure in the upper half of the macula. This finding was consistent with partial occlusion of the upper macular branch of the central retinal artery with confirmed presence of cilioretinal artery supplying the macular region. Possible association with surgery was excluded as this was beyond 14-days after decompression. Therefore, this must have been caused by a new microembolisation. After a consultation with cardiologists, vasodilatatory therapy was administered, consisting of intravenous infusion of normal saline solution with Oxantil (etophylline 160 mg and theophylline 40 mg in 2 ml; HBM Pharma, Martin, Slovakia) and pentoxiphylline 100 mg. Regression of the pathological finding was observed after the fifth infusion – macular edema in the left eye almost completely disappeared and visual acuity improved to 6/7.5 (20/25). Pentoxifylline 400 mg per day orally was added to the therapy. Regression of the optic nerve disc edema was present during the subsequent follow-up examinations performed after one and two months (Fig. 8, 9). However, optic nerve disc atrophy and associated permanent changes of perimeter were observed (Fig. 10).
Discussion
Etiology of the AION may be arteritic and non-arteritic. Arteritic optic neuropathy is also called giant cell arteritis, temporal arteritis or Horton disease. It is caused by a non-infectious inflammation affecting vascular wall and leading to its thickening, narrowing of the lumen and production of giant cells. Causes of non-arteritic form of optic neuropathy are unclear. Medical history of these patients, who are often younger than patients with arteritic neuropathy, often includes arterial hypertension, hypercholesterolemia, and sometimes smoking.
A specific visual nerve anatomy – so called “disc-at-risk“, characterized by small or completely missing physiological excavation of the disc, represents an ophthalmologic risk factor for the AION. Optic disc drusen and other vascular anomalies also represent predisposing factors for AION.
Vascular events (stroke, transient ischemic attack, peripheral thromboembolism) occur in 0.6 – 15% of patients after transcatheter closure of the PFO [7 – 9]. In the reported case of a female patient, a rare finding of two different vascular pathologies at the back of the left eye was observed four years after transcatheter PFO closure. This is consistent with the data reported in the literature, with arterial occlusion occurring on average five years after surgical PFO closure [7].
Although decompression of the optic nerve sheaths in AION patients remains controversial, this procedure represents one of the very few AION treatment options and our experience with this type of intervention is good [10] as also evidenced by the presented case, where this surgical procedure led to improved visual acuity. The aim of the intervention is to reduce cerebrospinal fluid pressure within the perineural subarachnoid space that could relieve the “compartment syndrome”, improve local blood flow and enhance axoplasmic flow within the damaged axons in AION [11]. The role of pharmacological treatment (oral steroids) remains controversial, and literature regarding prevention of sequential AION is contradictory. However, both treatment of vascular risk factors and antiplatelet therapy have an established role in the prevention of cerebral and myocardial infarction and should be also considered in AION [12]. Optic nerve disc edema occurring during intracranial hypertension or drusen of the optic nerve head are associated with axon damage that causes an increase in pressure inside the optic nerve sheaths. Nerve fibre damage may also be associated with other etiologies and normotensive axon destruction. Both mechanisms are then potentiated in the progressive form of the AION, causing severe optic nerve damage. Correct indication and timing of the procedure are the key prerequisites for its use. Surgeries performed at the time of self-limiting ischemic edema or even of partial transition to disc atrophy (with corresponding severely affected visual functions) have practically no chance for success. In our case, early diagnosis of the AION progression and early surgery resulted in improved visual functions and regression of the optic nerve disc edema three days after the surgery. Nevertheless, regression of the optic nerve disc edema during natural course of the disease takes longer than three days.
Neurologically, retinal cell death attributable to ischemia is included in the definition of central nervous system infarction. PFO is considered as treated after its transcatheter closure. According to the valid guidelines of the American Heart Association/American Stroke Association (ASA), there are insufficient data to establish whether anticoagulation is equivalent or superior to ASA for secondary stroke prevention in patients with PFO and, for patients with ischemic stroke or transient ischemic attack and PFO, who are not undergoing anticoagulation therapy, antiplatelet therapy isrecommended [13].
To conclude, we described a very rare case of occlusion of two different branches of the ophthalmic artery – central retinal artery (supplying the retina) and short posterior ciliary artery (supplying the optic nerve) with two different symptoms at the back of the eye (occlusion of the macular branch of the central retinal artery and the AION). Thus, two mechanisms, arterial occlusion after transcatheter PFO closure and vascular wall compression due to the optic nerve edema in AION, were involved. Possible association with surgery was excluded, because a 14-day interval passed from the date of decompression.
According to our knowledge, no such finding in a young female patient has been described so far. Regression of ischemic changes of the optic nerve disc and the retina, as well as improvement in visual acuity were observed following early initiation of an intensive vasodilation therapy and early decompression of the optic nerve sheaths. Functional cilioretinal artery supplying the macula ensured preservation of visual acuity, while incomplete recovery of visual functions was associated with initial atrophic changes of the optic nerve. Physicians must be aware of the risk of repeated embolization to various locations of the vascular system despite successful transcatheter PFO closure.
This case study was partially supported by the grant projects number FNHK 00179906, PRVOUK: P37/07 and PRVOUK: P37/08.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Assoc. Prof. Martin Valis, MD, PhD
Department of Neurology
Comprehensive Stroke Center
Faculty of Medicine
Charles University and University Hospital
Sokolska 581
500 05 Hradec Kralove
e-mail: valismar@seznam.cz
Accepted for review: 13. 12. 2015
Accepted for print: 19. 1. 2016
Sources
1. Hayreh SS. Anterior ischemic optic neuropathy: differentiation of arteritic from nonarteritic type and its management. Eye (Lond) 1990;4(1):25 – 41.
2. Johnson LN, Arnold AC. Incidence of nonarteritic and arteritic anterior ischemic optic neuropathy: population-based study in the state of Missouri and Los Angeles County, California. J Neuroophthalmol 1994;14(1):38 – 44.
3. Hayreh SS, Joos KM, Podhajsky PA, et al. Systemic diseases associated with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol 1994;118(6):766 – 80.
4. Tesser RA, Niendorf ER, Levin LA. The morphology of an infarct in nonarteritic anterior ischemic optic neuropathy. Ophthalmology 2003;110(10):2031 – 5.
5. Brown GC, Magargal LE, Shields JA, et al. Retinal arterial obstruction in children and young adults. Ophthalmology 1981;88(1):18 – 25.
6. Kim US, Kim HS, Lew YJ. A case of branch retinal artery obstruction complicated after anterior ischemic optic neuropathy. Int J Ophthalmol 2011;4(4):447 – 8. doi: 10.3980/ j.issn.2222-3959.2011.04.24.
7. Fischer D, Gardiwal A, Haentjes J, et al. Sustained risk of recurrent thromboembolic events in patients with patent foramen ovale and paradoxical embolism: long-term follow-up over more than 15 years. Clin Res Cardiol 2012;101(4):297 – 303. doi: 10.1007/ s00392-011-0392-2.
8. Windecker S, Wahl A, Nedeltchev K, et al. Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke. J Am Coll Cardiol 2004;44(4):750 – 8.
9. Schuchlenz HW, Weihs W, Berghold A, et al. Secondary prevention after cryptogenic cerebrovascular events in patients with patent foramen ovale. Int J Cardiol 2005;101(1):77 – 82.
10. Jirásková N, Rozsíval P. Výsledky 62 dekompresí obalů zrakového nervu. Cesk Slov Oftalmol 1999;55(3):136 – 44.
11. Arnold AC. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol 2003;23(2):157 – 63.
12. Atkins EJ, Bruce BB, Newman NJ, et al. Treatment of nonarteritic anterior ischemic optic neuropathy. Surv Ophthalmol 2010;55(1):47 – 63. doi: 10.1016/ j.survophthal.2009.06.008.
13. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/ American Stroke Association. Stroke 2014;45(7):2160 – 236. doi: 10.1161/ STR.0000000000000024.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2016 Issue 4
-
All articles in this issue
- Epilepsy Surgery Improves Quality of Life – Results of a Questionnaire Study
- Morphological Changes of Median Nerve in Patients with Newly Diagnosed, Untreated Autoimmune Hypothyroidism
- Surgical Principles of Dumbbell-shaped Spinal Nerve Sheath Tumors
- A Tubulopapillary Adenoma of the Gallbladder – Incidental Finding in a 3-year-old Boy later Diagnosed with Metachromatic Leukodystrophy – a Case Report
- Retinal Nerve Fiber Layer Measurement in Patients with Alzheimer’s Disease
- Neurorehabilitation of Sensorimotor Function after Spinal Cord Injury
- Genetic and Epigenetic Factors Affecting Development and Prognosis of Brain Gliomas – a Review of Current Knowledge
- Three Times of the Clock Drawing Test Rated with BaJa Scoring in Patients with Early Alzheimer‘s Disease
- Bicycle Drawing Test – Validation Study for Dementia
- Anterior Ischemic Optic Neuropathy and Branch Retinal Artery Occlusion after Transcatheter Closure of Foramen Ovale – a Case Report
-
Introduction to the Conference
Different Approaches in Neurorehabilitation and their Impact on Clinical Improvements of Neurological Patients
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Neurorehabilitation of Sensorimotor Function after Spinal Cord Injury
- Three Times of the Clock Drawing Test Rated with BaJa Scoring in Patients with Early Alzheimer‘s Disease
- Genetic and Epigenetic Factors Affecting Development and Prognosis of Brain Gliomas – a Review of Current Knowledge
- Retinal Nerve Fiber Layer Measurement in Patients with Alzheimer’s Disease
