Prevalence of Martin-Gruber Anastomosis – an Electrophysiological Study
Prevalence Martin-Gruberovy anastomózy – elektrofyziologie studie
Úvod:
Prevalence Martin-Gruberovy anastomózy (MGA), spojky n. medianus a ulnaris na předloktí se uvádí v rozmezí 15 – 39 %. Existují tři různé typy MGA, kdy motorická vlákna jsou v oblasti paže a lokte vedena skrze n. medianus a zásobují svaly ruky inervované n. ulnaris (m. abductor digiti minimi, m. interosseus dorsalis primus či m. adductor pollicis).
Soubor a metodika:
V pěti EMG laboratořích bylo unifikovanou technikou vyšetřeno 292 zdravých osob ve věku 20 – 67 let, průměr 39,4 let: 166 žen (256 rukou) a 126 mužů (201 rukou), celkem 457 rukou. Byla provedena motorická a senzitivní neurografie n. ulnaris a n. medianus. Pro detekci MGA mělo zásadní význam hodnocení amplitudy CMAP pro n. ulnaris a n. medianus při stimulaci z oblasti lokte a zápěstí.
Výsledky:
V našem souboru 457 vyšetřených rukou jsme na 90 rukou našli 109 výskytů MGA. U 30 rukou se jednalo o MGA-I, u 57 rukou o MGA-II a u 22 rukou o MGA-III. Izolované typy MGA se vyskytly v 73 případech, Na 17 rukou se vyskytla kombinace dvou, ojediněle dokonce všech tří typů MGA současně.
Závěr:
V souboru 292 osob zdravých osob jsme na 457 hodnocených rukou našli MGA v 19,7 %. Nejčastěji se vyskytoval typ MGA-II (12,5 %).
Klíčová slova:
Martin-Gruberova anastomóza – elektromyografie – nervus ulnaris – nervus medianus
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors:
E. Ehler 1; P. Ridzoň 2; P. Urban 3; R. Mazanec 4; H. Matulová 5; P. Otruba 6; P. Mandysová 1; M. Nakládalová 6
Authors‘ workplace:
st Faculty of Medicine, Charles
University and General University
Hospital in Prague
1; Department of Neurology, Faculty of
Health Studies, University of Pardubice
and Regional Hospital Pardubice
1; Department of Neurology, Thomayer
Hospital, Prague
2; Department of Occupational Medicine
3; Department of Neurology, 2nd Faculty
of Medicine, Charles University in
Prague and Motol University Hospital
Prague
4; Department of Neurology, Faculty
of Medicine, Charles University and
University Hospital Hradec Králové
5; Department of Neurology, Faculty
of Medicine and Dentistry, Palacký
University and University Hospital
Olomouc
6
Published in:
Cesk Slov Neurol N 2017; 80/113(4): 434-439
Category:
Original Paper
doi:
https://doi.org/10.14735/amcsnn2017434
Overview
Objective:
Martin-Gruber anastomosis (MGA) is a median-to-ulnar nerve communication in the forearm; three types of MGA occur. Typically, motor fibres course through the median nerve in the upper arm and elbow, however, they supply the ulnar-innervated muscles of the hand: abductor digiti minimi (ADM) – MGA-I; first dorsal interosseous (FDI) – MGA-II; or adductor pollicis – MGA-III. The objective was to determine the prevalence of MGA in a study group of healthy volunteers.
Methods:
Two hundred and ninety-two healthy participants (457 arms) were enrolled. Motor and sensory nerve conduction studies of the ulnar and median nerves were performed. Ulnar and median nerve compound muscle action potential amplitudes were obtained on stimulation at the elbow and wrist.
Results:
We found 109 cases of MGA in 90 arms (MGA-I in 30 arms; MGA-II in 57 arms; MGA-III in 22 arms). We found isolated MGA types in 73 arms, a combination of two types in 15 arms, and occasionally (2 arms) a simultaneous combination of all three types.
Conclusion:
The prevalence of MGA was 19.7%. Most frequently, we found MGA-II (prevalence = 12.5%).
Significance:
MGA does not produce any clinical signs. However, it can change EMG results. The neurophysiologist must be able to logically interpret such findings.
Key words:
Martin-Gruber anastomosis – electromyography – ulnar nerve – median nerve
Chinese summary - 摘要
Martin-Gruber吻合支的患病率 - 一项电生理研究目的:
马格二世吻合术(MGA)是前臂正中神经与尺神经的一种神经连通术,当前有三种类型。 通常,运动纤维穿过上臂和肘部的正中神经。然而,它们支配着手部的尺神经肌肉:包括拇指展肌(ADM)-MGA-I;第一背侧骨间(FDI)-MGA-II或拇内收肌-MGA-III。本研究试图确定健康人群中MGA的患病率。
方法:
本次共招募了292位健康被试(457支手臂),进行尺神经和正中神经的运动和感觉神经传导研究。尺神经和正中神经复合肌肉动作电位振幅由手肘和腕部刺激获得。
结果:
我们在90支手臂(MGA-I 30支,MGA-II 57支,MGA-III 22支)中发现了109例MGA。我们还在73支手臂中发现了单独的MGA类型,其中15支手臂是两种类型的组合,偶尔(2支手臂)同时出现了三种类型的组合。
结论:
MGA患病率为19.
7%, 其中MGA-II型最常见(患病率= 12.5%)。
意义:
MGA没有任何临床症状,但是它可以改变肌电图的结果。神经生理学家一定能够从逻辑上解释这些发现。
关键词:
Martin-Gruber吻合支 - 肌电图 - 尺神经 - 正中神经
Introduction
Anomalous innervation arising from the median nerve (MN) and the ulnar nerve (UN) was first described by Martin in 1763 and later by Gruber in 1870. In Martin-Gruber anastomosis (MGA), some of the motor fibres that course through the MN branch off in the proximal forearm and join the UN. MGA is the most common anastomosis between both nerves; the reported prevalence is 15 – 30%. Its occurrence may have a hereditary basis, consistent with an autosomal dominant pattern of inheritance [1].
Three types of MGA occur, which change the classic motor conduction study findings:
MGA-I: The MN fibres that have crossed over terminate in the abductor digiti minimi (ADM), which they partially innervate. UN motor conduction study with recording from the ADM results in a higher amplitude of the compound muscle action potential (A-CMAP) on stimulation of the UN at the wrist than on stimulation at the elbow, where the fibres have not yet crossed over from the MN. If the difference between the amplitudes exceeds 20% (or 2 mV), the presence of the anastomosis is confirmed. This finding can be interpreted incorrectly as a conduction block of the UN. The electromyography specialist verifies the presence of MGA-I (Fig. 1) by stimulating the MN at the elbow and recording the compound muscle action potential (CMAP) over the ADM.

MGA-II: Motor fibres from the MN cross over in the upper forearm, join the UN, and partially innervate the first dorsal interosseous muscle (FDI). On stimulation of the UN at the elbow, the amplitude of the CMAP obtained from the FDI is more than 20% (or 2 mV) lower compared with stimulation of the UN at the wrist. This finding can be interpreted incorrectly as a conduction block of the UN. However, recording the CMAP over the FDI on stimulation MN at the elbow confirms the presence of MGA-II (Fig. 2).

MGA-III: Motor fibres from the MN cross over in the upper forearm, join the UN, and supply the adductor pollicis and the deep head of the flexor pollicis brevis (Fig. 3). Motor nerve conduction study of the MN reveals that the amplitude of the CMAP obtained from the thenar eminence on stimulation of the MN at the wrist is lower compared with stimulation of the MN at the elbow. Because stimulation of the adductor pollicis occurs faster via the UN than stimulation of the abductor pollicis brevis (APB) via the MN, this stimulation of UN evokes a thenar CMAP with a positive deflection. Calculation of the MN conduction velocity gives a markedly high value if it is based on this deflection. It is possible to verify the presence of the anastomosis by stimulating the UN; this stimulation evokes a thenar CMAP that has a much higher amplitude on stimulation of the UN at the wrist than at the elbow. If the difference between the amplitude of the CMAP on stimulation of the MN at the elbow and wrist exceeds 10%, then a diagnostic criterion for MGA-III is present.

In studies involving human corpses, the overall prevalence of MGA was in 11 – 24% of the cases. In earlier conduction studies involving the limbs, the prevalence was 15 – 39% [2]. Martin-Gruber anastomosis has its importance in clinical practice; in cases of MN lesion, the presence of this anastomosis may mimic a (partial) lesion of the UN as well. Electrophysiological studies reveal a larger number of abnormal parameters, which can lead to the wrong diagnosis of conduction block in the elbow segment of the UN or to the incorrect diagnosis of carpal tunnel syndrome.
In our normative study of MN and UN conduction [3], we examined the occurrence of MGA as well. In this paper, we present our results.
Methods
This was a multicentre study conducted in five EMG laboratories in the Czech Republic employing a uniform method and using the Medelec Synergy EMG System.
Two hundred and ninety-two healthy volunteers underwent motor and sensory nerve conduction studies of the UN and MN. The study group included 166 women and 126 men aged 20 – 67 years (average age of 39.4 years). Arms with abnormal findings were excluded (polyneuropathy, MN lesions in the carpal tunnel, and UN lesions in the region of cubital tunnel or in Guyon’s canal). Furthermore, some of the participants gave their consent to a study involving only one upper extremity. Therefore, the resulting sample included 457 healthy arms (256 female arms and 201 male arms).
The study was conducted by means of common surface electrodes supplied by the manufacturer. Medelec Synergy EMG System surface stimulation electrodes were used. Motor conduction was recorded with a disposable surface electrode, and sensory conduction was recorded using a ring electrode with conductive gel. For motor neurography, the stimulus duration was set to 0.2 ms; for sensory neurography, it was set to 0.1 ms. Filters were from 3 to 10 kHz for motor neurography and from 20 to 2 kHz for sensory neurography.
The MN was stimulated at the cubital fossa and at the wrist; it was recorded from the APB. The distance between the cathode of the stimulation electrode at the wrist and the active electrode above the APB was 8 cm (measured at an angle). The UN was stimulated at the wrist, below the elbow, and above the elbow. Recording electrodes were placed over ADM and FDI. The examination was conducted with the extremity in a standard position, in semiflexion of the elbow at a 90° angle. The distance between the cathode of the stimulation electrode at the wrist and the active electrode above ADM was 8 cm, and it was 13 cm to the active electrode above FDI (measured at an angle). Stimulation of the UN below the elbow was located 4 cm distally from the medial epicondyle. Stimulation above the elbow was placed 6 cm proximally from the medial epicondyle of the humerus.
Sensory nerve conduction was measured antidromically. For the MN (on the 2nd digit), the distance between the stimulation and recording electrode equalled 16 cm; for the UN (on the 5th digit), it was 14 cm. Individual recordings were averaged. Latency was measured to the onset of the negative deflection, and amplitude to its peak. Limb temperature at the base of the 4th digit was at least 32 degrees Celsius (°C).
MGA detection
MGA-I was detected based on a drop in CMAP amplitude in ADM (more than 20%) on stimulation in the elbow region compared with stimulation at the wrist. We selected the value of 20% in accordance with Uchida and Sugioka [4], Oh [5] and Preston and Shapiro [6].
MGA-II was detected based on a drop in CMAP amplitude in FDI (more than 20%) on stimulation in the elbow region compared with stimulation at the wrist. We selected the value of 20% in accordance with authors [4 – 6].
MGA-III: If on stimulation at the wrist, the amplitude of MN CMAP was 10% lower compared with amplitude obtained on stimulation in the elbow region, the finding was determined to be MGA-III.
Results
In our sample of 457 examined arms, one of the MGA types was found in 90 arms, which corresponds to a prevalence of 19.7%. In these 90 arms, we found a total of 109 cases of MGA, which were present either as isolated, single types or as various combinations of the MGA types occurring simultaneously in a given arm (Fig. 4, Tab. 1).

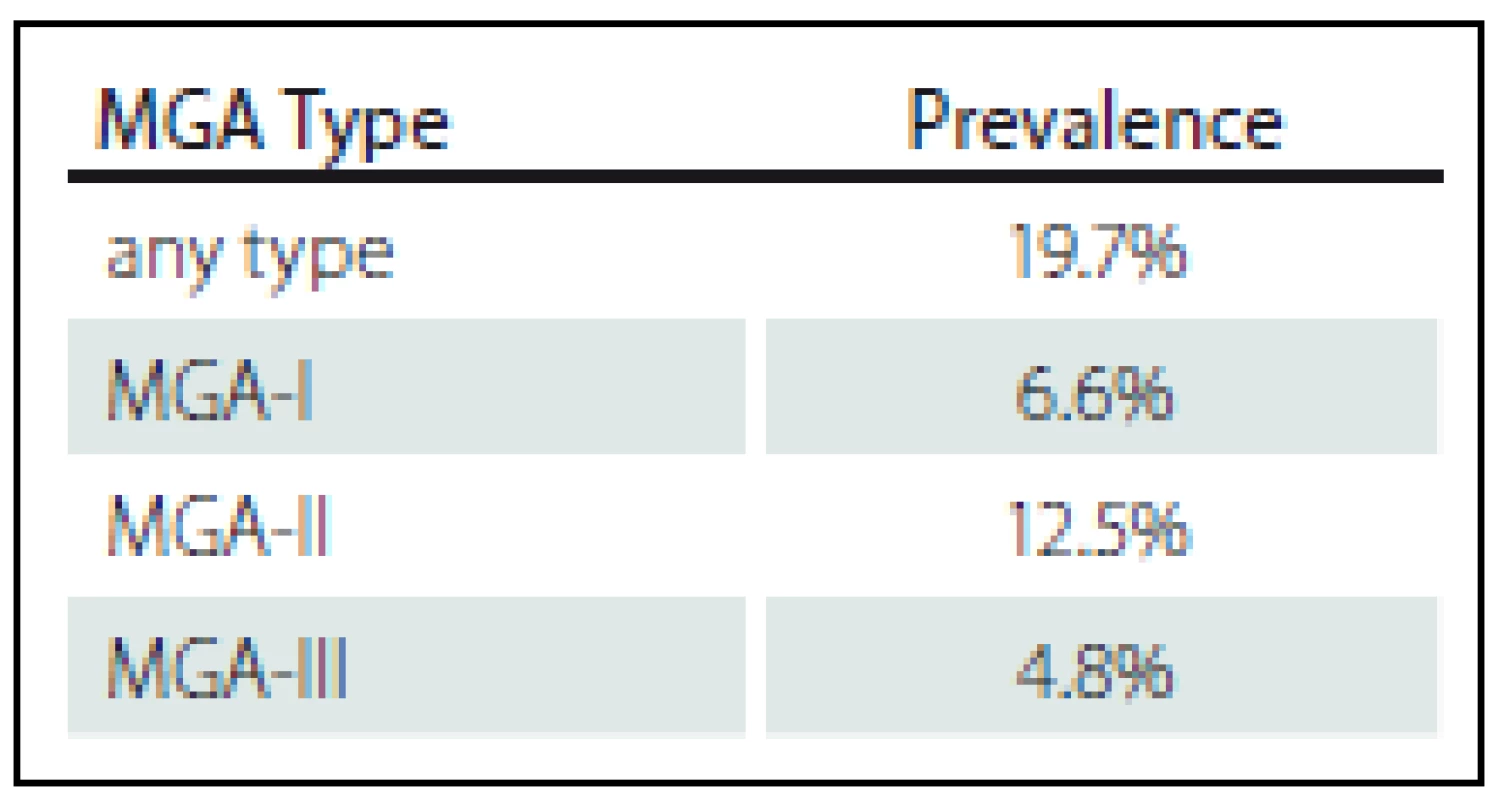
MGA-I was found in 30 cases (a prevalence of 6.6% out of 457 arms), MGA-II was found in 57 cases (12.5% out of 457 hands), and MGA-III was found in 22 arms (4.8% out of 457 arms). Isolated, single MGA types (MGA-I, MGA-II, or MGA-III) were present in 73 cases (16% out of 457 arms). A combination of two types, and occasionally a simultaneous combination of all three MGA types was found in 17 arms (3.7% out of 457 arms). MGA-II was the most frequently occurring MGA type (Fig. 4).
Discussion
A number of anatomical studies have demonstrated the presence of crossover innervation from the MN to the UN by means of various kinds of anastomoses that contained only motor fibres. The anastomosing fibres can travel through the anterior interosseous nerve or through the anastomosis to the flexor digitorum profundus. Lee et al. analysed a sample of 102 arms and found MGA in 40 of the arms (i.e., in 39% of the cases) [7]. Loukas et al. studied the anastomosis between both nerves in the region of the proximal forearm [8]. The anastomosis measured 1 – 1.5 mm in diameter and its length was 2.5 cm. Wilbourn and Lambert found that in a group of 22 persons with confirmed MGA, anastomotic fibres innervating the ADM (MGA-I), the FDI (MGA-II), and the thenar eminence (the adductor pollicis muscle) (MGA-III) were present in 9, 21 and 3 cases, resp. [9]. In their anatomical study, Felippe et al. examined 30 forearms [10]. They found 3 cases of MGA (the average length of the anastomosis was 6.6 cm) and a 7.4 cm long Marinacci anastomosis. Paulos and Leclercq dissected 20 frozen human cadaver arms and examined them under a microscope; MGA was found in 9 of the cases [11].
Based on the anatomical findings, it is recommended to stimulate the UN at a site that is no more than 3 – 4 cm distal from the intercondylar line. In our study, the stimulation site was 4 cm distal from this line.
Uchida and Sugioka [4] determined that the criterion for electrophysiological diagnosis of MGA-I and MGA-II consists in the presence of A-CMAP reduction on UN stimulation below the elbow by 20% compared to A-CMAP on stimulation at the wrist. Lee et al. believe that MGA (all three types) is present even if A-CMAP reduction is as small as 1mV (i.e. 5 – 10%) [7]. Claussen et al. determined that for all three types of MGA, an amplitude reduction of 25% was borderline [12]. However, according to Kate et al., even a 10% A-CMAP reduction is significant. In our study, the diagnosis of MGA-I and MGA-II was made if there was a 20% A-CMAP reduction on UN stimulation in the elbow region (distally and proximally) [13].
Electrophysiological diagnosis of MGA-III is based mainly on conduction studies of the motor fibres in the MN. This particular MGA type is the least frequent. Anatomically, this involves the thenar eminence on stimulation of the UN (the adductor pollicis and the deep head of the flexor pollicis brevis). Motor nerve conduction studies of the MN reveal that on stimulation of the MN at the wrist, the obtained amplitude of the CMAP is lower by at least 10% compared with stimulation at the elbow. In some cases, higher motor nerve conduction velocity (MNCV) in the forearm is observed (Preston and Shapiro) [6]. Katirji reports a reduction of the A-CMAP by 10 – 20% [14]. For the presence of MGA-III, it is typical to encounter an initial positive deflection (a dip) on UN stimulation at the wrist (but not on stimulation at the elbow). On stimulation MN at the elbow, the CMAP has two peaks (a “double-hump”) [15]. Distal motor latency has normal values and proximal latency is shorter because the fibres with the fastest conduction run through the UN rather than through the carpal tunnel. Most authors emphasise that establishing the diagnosis of MGA-III is difficult.
In our work, the criterion for MGA-III consisted of at least a 10% reduction of A-CMAP at the wrist in comparison with A-CMAP on stimulation at the elbow. Other accompanying abnormalities such as the initial dip, a double-hump, higher MNCV of the MN at the forearm, were rarely observed in our study group.
MGA containing motor and sensory fibres with sensory manifestations is extremely rare [12]. However, sensory neurography based on the near-nerve needle technique detects MGA through sensory nerve action potential (SNAP) quite frequently. Simonetti found a low SNAP on stimulation of the MN in 10 of the 24 examined arms [16]. In nine of them, however, SNAP disappeared on infiltration of the UN in the elbow region using lidocaine. This represented a volume effect.
A number of authors have studied the occurrence of MGA using electrodiagnostic studies. In a study conducted by Uchida and Sugioka, 134 individuals were enrolled, and MGA was found in 22 (16%) of the cases [4]. The entry point of the fibres from the MN to the UN was 3 – 10 cm distal to the medial humeral epicondyle. Hasegawa et al. examined 106 healthy individuals [17]. Using motor neurography, they recorded CMAP over the ADM, FDI, and adductor policis. In all cases, they stimulated both nerves, at the wrist and above the elbow. MGA-I was found in 11% of the cases. MGA-II and MGA-III were found in 25% and 11% of the cases, resp.
Kayamori performed EMG testing of 600 healthy subjects (1,200 upper arms) and found MGA in 83 (14%) individuals, namely in 116 (8.3%) upper arms [18]. Bilateral MGA was present in 33 (40%) individuals, and unilateral MGA was found in 50 (60%) individuals; in 33 individuals, it was present in the right upper extremity, and in 17 individuals, it was present in the left upper extremity. Bertorini identified MGA in 31% of the studied individuals [15]. In 62% of the cases, MGA was present in individuals who were related to each other. Bilateral MGA occurred in 68% of the cases. To demonstrate its presence, the collision technique was used to conduct motor stimulation. The method employed by us to examine the UN was almost identical to that of Acosta et al. [19]. In one of their four patients with diabetes mellitus, they focused on the exclusion of MGA as the cause of CMAP amplitude reduction with UN stimulation. Amoiridis examined 100 healthy individuals and found MGA in 32% of the cases [20]. In a study conducted by Lee et al., 102 individuals were enrolled, and MGA was found in 39.2% of the cases [7]. In 12 of them, the anastomosis passed through the branches to reach the flexor digitorum profundus. Erdem et al. identified MGA in 27 out of 100 volunteers [21]. MGA-I and MGA-II were present in 3 and 21 subjects, resp; MGA-III was found in 1 subject, and a combination of type I and type II was found in 2 of the cases. Crutchfield and Gutmann examined 50 healthy individuals (100 arms); they confirmed the presence of MGA (in at least one arm) in 14 (28%) of the individuals tested; bilateral MGA was found in 9 out of these 14 volunteers [1]. In addition, family members of these individuals were examined, with the aim of detecting a presence of MGA. It was determined that the data supported an autosomal dominant mode of inheritance (mutations were not studied).
Roy et al. conducted a meta-analysis of 58 articles concerning MGA prevalence [22]. In these studies, a total of 10,562 upper extremities were examined. Based on these data, pooled MGA prevalence in the population was 19.5% (95% confidence interval; CI: 16.2 – 23.1%).
In our study, we examined 457 healthy upper extremities. In 90 (19.7%) of the arms, we found 109 cases of MGA. Most of the identified cases (52% out of the 109 cases) represented MGA-II, which was in line with the results of other authors. MGA-I was present in 28% and MGA-III in 20% of the cases. A comparison of our results and the results of other authors is summarised in Tab. 2.
![A comparison of our results with the results of other authors [1,4,10,15,18,20–22,29].](https://www.csnn.eu/media/cache/resolve/media_object_image_small/media/image/0181f97c15cfc798ae50b9c664bc7d2f.png)
Ascertaining the presence of MGA and considering its presence during an interpretation of neurophysiological findings is a task performed by an electromyography specialist [23]. Most frequently, MGA is erroneously interpreted as a UN lesion in the elbow region – a reduction of CMAP amplitude due to conduction block [24,25]. To obtain a correct diagnosis, the examination site should be distal from the medial epicondyle (however, not exceeding 3 cm) and simultaneously, MN stimulation is performed [26]. Mistaking a MGA for another clinical disorder can have undesired effects (an operation in the elbow region, additional unnecessary testing – ultrasound, magnetic resonance imaging) [27,28].
Conclusion
MGA is a fairly frequent finding. Clinicians, and especially electromyography specialists, need to be aware of this anomalous innervation (the diagnostic process, indications for carpal tunnel surgery, or in relation to trauma, as the case may be). In MGA-I and MGA-II, the reduction of CMAP amplitude on stimulation of the UN in the elbow region can mimic conduction block. In MGA-III, the reduction of CMAP amplitude on MN stimulation at the wrist can lead to diagnostic uncertainty or can raise suspicion of abnormal course of the MN. However, other characteristics – shorter motor latency on proximal stimulation, positive CMAP deflection, CMAP recording from the thenar eminence on stimulation of the UN – constitute the correct path to the diagnosis. In our study, based on a group of individuals enrolled in five centres across the Czech Republic, we have revealed considerable prevalence of MGA. Thus, we have demonstrated that MGA prevalence in the Czech population does not differ from the prevalence seen in other countries and continents. The presence of Martin-Gruber anastomosis does not produce any clinical manifestations. However, the identification of MGA and the determination of its type can be done by using EMG – it is a task performed by the electromyography specialist – the neurophysiologist.
Highlights
Connections between the MN and UN in the upper part of the forearm are the most common form of anastomosis involving peripheral nerves. The anastomosis is present when some of the motor fibres innervating the hand muscles in the ulnar region are found as far as the elbow in the MN nerve.
Our sample of all three types of MGA was quite large; the data were collected in five different electromyography (EMG) laboratories that used a uniform methodology for the examination of both nerves.
The presence of MGA does not produce any clinical manifestations. However, the identification of MGA and the determination of its type can be done by using EMG; it is a task performed by the electromyography specialist – the neurophysiologist.
List of used abbreviations
APB – abductor pollicis brevis
A-CMAP – amplitude of compound muscle action potential
ADM – abductor digiti minimi
CMAP – compound muscle action potential
DML – distal motor latency
MGA – Martin-Gruber anastomosis
EMG – electromyography
FDI – first dorsal interosseous
MN – median nerve
MNCV – motor nerve conduction velocity
SNAP – sensory nerve action potential
UN – ulnar nerve
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
assoc. prof. Edvard Ehler, MD, CSc.
Department of Neurology
Faculty of Health Studies
University of Pardubice and Regional Hospital Pardubice
Kyjevská 44
532 03 Pardubice
e-mail: eda.ehler@tiscali.cz
Accepted for review: 3. 2. 2017
Accepted for print: 2. 5. 2017
Sources
1. Crutchfield CA, Gutmann L. Hereditary aspects of median-ulnar nerve communications. J Neurol Neurosurg Psychiatry 1980;43(1):53 – 5.
2. Burakgazi AZ, Russo M, Bayat E, et al. Ulnar neuropathy with prominent Martin-Gruber anastomosis. Int J Neurosci 2014;124(7):542 – 6. doi: 10.3109/ 00207454.2013.858336.
3. Ehler E, Ridzoň P, Urban P, et al. Ulnar nerve at the elbow – normative nerve conduction study. J Brachial Plex Peripher Nerve Inj 2013;8(1):2. doi: 10.1186/ 1749-7221-8-2.
4. Uchida Y, Sugioka Y. Electrodiagnostic of Martin-Gruber connection and its clinical importance in peripheral nerve surgery. J Hand Surg 1992;17A:54 – 9.
5. Oh SJ. Clinical electromyography. Nerve conduction studies. Baltimore: Williams & Wilkins 1993 : 314 – 37.
6. Preston DC, Shapiro BE. Electromyography and neuromuscular disorders. London: Elsevier 2015 : 62 – 70.
7. Lee KS, Oh CS, Chung IH, et al. An anatomic study of the Martin-Gruber anastomosis: Electrodiagnostic implications. Muscle Nerve 2005;31(1):95 – 7.
8. Loukas M, Abel N, Tubbs RS, et al. Neural interconnections between the nerves of the upper limbs and surgical implications. J Neurosurg 2011;114(1):225 – 35. doi: 10.3171/ 2010.3.JNS10144.
9. Wilbourn AJ, Lambert EH. The forearm median to ulnar nerve communication: electrodiagnostic aspects. Neurology 1976;26 : 368.
10. Felippe MM, Telles FL, Soares ACL, et al. Anastomosis between median nerve and ulnar nerve in the forearm. J Morphol Sci 2012;29(1):23 – 6.
11. Paulos R, Leclercq C. Motor branches of the ulnar nerve to the forearm: an anatomical study and guidelines for selective neurectomy. Surg Radiol Anat 2015;37(9):1043 – 8. doi: 10.1007/ s00276-015-1448-1.
12. Claussen GC, Ahmad GK, Sunwood IN, et al. Combined motor and sensory median-ulnar anastomosis: report of an electrophysiologically proven case. Muscle Nerve 1996;19(2):231 – 3.
13. Kate NN, Teli CG, Gajbhiye R, et al. A study to analyse the prevalence of nervous anastomosis (Martin-Gruber) in medical students. Natl J Physiol Pharm Pharmacol 2015;5(3):185 – 9. doi: 10.5455/ njppp.2015.5.1207201414
14. Katirji B, Kaminski HJ, Ruff LR. Neuromuscular disorders in clinical practice. New York: Springer 2014.
15. Bertorini TE. Neuromuscular case studies. Memphis: Elsevier 2008 : 112 – 5.
16. Simonetti S. Electrophysiological study of forearm sensory fibre crossover in Martin-Gruber anastomosis. Muscle Nerve 2001;24(3):380 – 6.
17. Hasegawa G, Matsumoto S, Lino M, et al. Prevalence of Martin-Gruber anastomosis on motor nerve conduction studies. Brain Nerve 2001;53(2):161 – 4.
18. Kayamori R. Electrodiagnosis of Martin-Gruber anastomosis. J Jpn Orthop Assoc 1987;61(12):1367 – 72.
19. Acosta JA, Hoffman SN, Raynor EM, et al. Ulnar neuropathy in the forearm: a possible complication of diabetes mellitus. Muscle Nerve 2003;28(1):40 – 5.
20. Amoiridis G. Median-ulnar nerve communications and anomalous innervation of the intrinsic hand muscles: An electrophysiological study. Muscle Nerve 1992;15(5):576 – 9.
21. Erdem HR, Ergun S, Erturk C, et al. Electrophysiological evaluation of the incidence of martin-gruber anastomosis in healthy subjects. Yonsei Med J 2002;43(3):291 – 5.
22. Roy J, Henry BM, Pekala PA, et al. Median and ulnar nerve anastomoses in the upper limb: a meta-analysis. Muscle Nerve 2016;54(1):36 – 47. doi: 10.2002/ mus.24993
23. Amoiridis G, Vlachonikolis IG. Verification of the median-to-ulnar and ulnar-to-median nerve motor fiber anastomosis in the forearm: an electrophysiological study. Clin Neurophysiol 2003;114(1):94 – 8.
24. Van Dijk JG. Anomalies of innervation. In: Kimura J, ed. Handbook of clinical neurophysiology. Vol. 7.Peripheral nerve diseases. Edinburgh: Elsevier 2006 : 311 – 44.
25. Van Dijk JG, Bouma PA. Recognition of the Martin-Gruber anastomosis. Muscle Nerve 1997;20(7):887 – 9.
26. Marras C, Midroni G. Proximal Martin-Gruber anastomosis mimicking ulnar neuropathy at the elbow. Muscle Nerve 1999;22(8):1132 – 5.
27. Kayamori R. Electrodiagnosis of Martin-Gruber anastomosis. J Jpn Orthop Assoc 1987;61(12):1367 – 72.
28. Whitaker CH, Felilce KJ. Apparent conduction block in patients with ulnar neuropathy at the elbow and proximal Martin-Gruber anastomosis. Muscle Nerve 2004;30(6):808 – 11.
29. Khosrawi S, Klanimehr I, Andalib S. The prevalence of Martin-Gruber anastomosis in Iranian subjects by electrodiagnostic criteria. Iran J Neurol 2015;14(4):231–2.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2017 Issue 4
-
All articles in this issue
- Ataxia
- Patient with Hemiplegia Should be Transported Right to the Cerebrovascular Center
- Patient with Hemiplegia Should not be Transported Right to the Cerebrovascular Center
- Should be Patient with Hemiplegia Transported Right to the Cerebrovascular Center?
- Cognitive Functions in Low-grade Glioma Patients – a Systematic Review
- Clinical Importance of Radiological Parameters in Lumbar Spinal Stenosis
- Neurosonological Markers Predict ing Cognitive Deterioration
- Czech National Guillain-Barré Syndrome Registry
- The Role of Drug-induced Sleep Endoscopy in Treatment (Surgical and Non-surgical) in Patients with Obstructive Sleep Apnea
- Nerve Injuries in Supracondylar Humeral Fractures in Children
- A Comprehensive Nationwide Evaluation of Stroke Centres in the Czech Republic Performing Mechanical Thrombectomy in Acute Stroke in 2016
- Clinical View of the Otorhinolaryngologist and Radiologist on the Classification of Fractures of the Temporal Bone
- Experience with using the RevoLix Jr thulium laser – Case Reports
- Dissection of All Four Cervical Arteries in a Patient with Fibromuscular Dysplasia – a Case Report
- Intravenous Thrombolysis after Dabigatran Reversal with a Specific Antidote Idarucizumab
- The Czech Pneumological and Physiological Society and the Czech Society for Paediatric Pulmonology Guidelines for Long-term Home Treatment Using the CoughAssist Machine in Patients with Serious Cough Disorders
- Prevalence of Martin-Gruber Anastomosis – an Electrophysiological Study
- Mortality Prediction in a Neurosurgical Intensive Care Unit
- The Effect of Different Occupational Therapy Techniques on Post-stroke Patients
- Comment of Article The Effect of Different Occupational Therapy Techniques on Post-stroke Patients
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Czech National Guillain-Barré Syndrome Registry
- Clinical View of the Otorhinolaryngologist and Radiologist on the Classification of Fractures of the Temporal Bone
- The Czech Pneumological and Physiological Society and the Czech Society for Paediatric Pulmonology Guidelines for Long-term Home Treatment Using the CoughAssist Machine in Patients with Serious Cough Disorders
- Nerve Injuries in Supracondylar Humeral Fractures in Children
