Aseptická meningitida při akutní hepatitidě E – zkušenosti z jednoho centra
Authors:
M. Mihalčin 1,2; M. Tvrdá 2; P. Vašíčková 3; P. Husa 1,2
Authors place of work:
Faculty of Medicine, Masaryk University, Brno, Czech Republic
1; Department of Infectious Diseases, University Hospital Brno, Czech Republic
2; Veterinary Research Institute, Brno, Czech Republic
3
Published in the journal:
Cesk Slov Neurol N 2021; 84(6): 572-573
Category:
Dopisy redakci
doi:
https://doi.org/10.48095/cccsnn2021572
Dear Editor,
We would like to describe two rare cases of CNS involvement (aseptic meningitis) in immunocompetent patients with acute hepatitis E, treated at the Department of Infectious Diseases, University Hospital Brno, Czech Republic.
In the last decade, it has been recognized that hepatitis E virus (HEV) is also endemic in the CR with the incidence of about 2.6/100 000 inhabitants [1]. Most cases of hepatitis E in Europe arise from infected animals such as pigs, wild boar, deer and rabbits. Zoonotic HEV genotypes (HEV genotypes 3–8) are mainly food-borne or transmitted by direct contact, but recent data suggest that infection can also be water-borne or iatrogenic through contaminated blood products [2].
Most patients with acute hepatitis E have no symptoms or the symptoms are indistinguishable from other forms of acute viral hepatitis. There have been several extrahepatic manifestations reported with hepatitis E [3–6]. Case reports and case series indicate that 5.5% of HEV-infected patients also have neurological manifestations such as Parsonage Turner syndrome, Guillain-Barré syndrome and encephalitis [3]. According to another analysis, the most often described ones were Guillain-Barré syndrome, Parsonage-Turner syndrome and multiplex mononeuropathy [4]. A French prospective study on 200 cases of acute hepatitis E and 200 controls found that 16.5% of infected patients showed neurological manifestations. The most frequent manifestations were neuropathic pain, painless sensory disorders, Parsonage-Turner syndrome, Guillain-Barre syndrome, encephalitis and meningitis [5]. There are also reports of vestibular neuritis, Bell’s palsy, acute ataxic neuropathy, transverse myelitis, acute encephalic Parkinsonism, oculomotor palsy, myositis, pseudotumor cerebri, bilateral pyramidal syndrome, polyradiculoneuropathy, mononeuritis multiplex, encephalitis and meningitis during or following HEV infection [6].
We present two cases of neurological involvement in patients with acute hepatitis E, hospitalized from 2018–2020. During the period, a total number of 19 patients were admitted with acute hepatitis E. We tested all of them to exclude hepatitis A, B and C. Concurrently we used the ELISA HEV IgG and IgM kits (both Dia.Pro company, Italy) to prove the positivity of anti-HEV antibodies and RealStar ®HEV RT-PCR Kit 1.0 (Altona Diagnostics, Germany) to detect the presence of HEV RNA. To exclude other causes of central nervous system inflammation, both patients were tested serologically for neuroborreliosis, tick-borne encephalitis, presence of anti-measles, anti-rubella, anti-varicella and zoster virus antibodies (MRZ reaction) and using the PCR method for the presence of herpes simplex virus 1 and 2, varicella and zoster virus and enteroviruses in the cerebrospinal fluid (CSF). Additional peripheral blood serological tests to rule out syphilis, HIV, or acute EBV and CMV infection were performed. In both cases of neurological involvement, serum concentration of C-reactive protein, bilirubin concentration and full blood count were in the range of normal values, which rules out the possibility of leptospirosis. None of the two patients did travel outside the Czech Republic in the last 6 months before the onset of the symptoms.
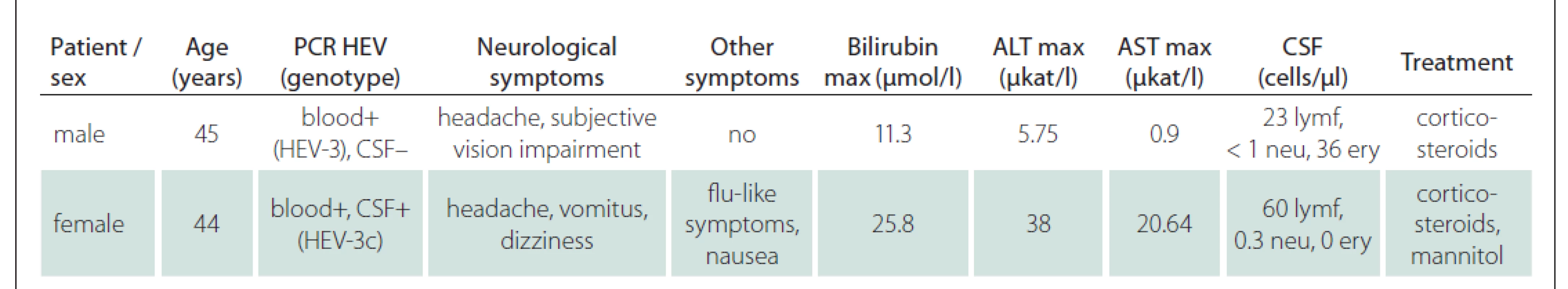
A 45-year-old male, chronically treated for type 2 diabetes and arterial hypertension, was admitted to the hospital 8 days after the onset of headache and subjective slower visual focusing. However, no objective visual disorder or any pathological findings were present in the neurological examination. After normal findings on a brain CT scan and CTA of the brain arteries, a lumbar puncture was performed and meningitis was confirmed (23 lymphocytic cells/μL, less than 1 neutrophil/μL, 36 erythrocytes/μL, total protein 0.334 g/L, 2 oligoclonal bands of the same type found in serum and CSF). Because of elevated liver enzymes upon admission (ALT 5.75, AST 0.9, GGT 7.2 µkat/L), viral hepatitis serologies were performed, positive for anti-HEV IgM and IgG antibodies. The test was confirmed by PCR detection of the HEV genotype 3 in the blood, but not in the CSF. Dexamethasone (8 mg t.i.d. for two days then 8 mg b.i.d. for two days and 8 mg on the fifth day) was administered for 5 days as a symptomatic treatment of serous meningitis, and then discontinued, with no other treatment used. All the symptoms disappeared during 8 days of hospital stay and did not recur until one and a half years after the infection.
A 44-year-old female was referred to our department with fever, fatigue, arthralgia, nausea and abdominal pain lasting for 4 days, with significantly elevated serum aminotransferase activity (ALT 38, AST 20.64, GGT 6.87 µkat/L), and with a slightly elevated serum bilirubin concentration (25.8 µmol/L). Her medical history included migraine with one attack per month, and she had not traveled abroad for a long time. On the day of admission, she started to complain about a severe headache, which was different than she was used to, while having a migraine attack. She was vomiting and felt unsteady. Physical examination and the ultrasound of the abdomen showed no significant abnormalities. A lumbar puncture was performed, and CSF revealed aseptic meningitis with increased lymphocytic cells count (60/µL), normal neutrophils (0.3/µL), and a higher level of total protein (1.03 g/L). A serological blood test detected anti-HEV IgM antibodies with anti-HEV IgG antibodies. Following confirmation using the PCR method proved RNA HEV presence in the blood and stool. Oligoclonal bands of the same type were detected in the serum and CSF, while the MRZ reaction was negative. MRI of the brain and cervical spine did not show any pathology. The patient was treated with dexamethasone same dosing as above, but 8 mg dose until day seven and mannitol. After corticosteroid administration, the headache was relieved immediately. Regular neurological examination showed normal findings. Liver enzyme serum activity decreased rapidly in the days following admission and corticosteroid administration. After 16 days, the patient was discharged with a serum aminotransferase activity of ALT 1.35 µkat/L, AST 0.38 µkat/L, GGT 3.18 µkat/L and bilirubin concentration 8.4 µmol/L. HEV was subsequently detected in the CSF also in a very small viral load. The virus sequencing confirmed the HEV-3c genotype. At the follow-up visit a month after discharge, the patient did not mention recurrence of any problems, liver functions were improved, with ALT and AST serum activity in the normal range and GGT slightly elevated (1.30 µkat/L).
Possible extrahepatic manifestations of HEV infection were noted in two patients with acute hepatitis E, as summarized in Tab. 1. These rare cases presented as uncomplicated aseptic meningitis. Both cases were confirmed by HEV RNA detection in the blood or stool. HEV RNA detection in the CSF was positive in one of the two cases. Other causes of infections of the CNS were ruled out by serologic testing (neuroborreliosis, tick-borne encephalitis, herpes simplex virus 1 and 2, varicella and zoster virus, syphilis, hiv human immunodeficiency virus, acute Epstein–Barr virus and cytomegalovirus infection) and serum concentration of C-reactive protein, bilirubin concentration and full blood count within the range of normal values ruled out the possibility of leptospirosis. Both cases were followed up in the consecutive year and no other explanation or ongoing neurological symptoms have been found. In both cases, corticosteroids were used as part of the treatment (beginning with dexamethasone 8 mg t.i.d. and lowering the dose for 7 days), as there is no evidence-based recommendation for the treatment of CNS complications of acute hepatitis E, and we regularly use such a protocol in the treatment of other viral CNS infections, where there is no causal treatment available. Ribavirin therapy to suppress viral replication does not outweigh the risks of administration, as the HEV infection is self-limited in most cases. Both our cases recovered without sequelae.
Although rare, there are various neurological syndromes during acute hepatitis E infection described in the literature, often seen in patients without signs of hepatitis. Despite the nomenclature of the virus, it is important to emphasize that patients with HEV-associated neurological injury do not usually present with jaundice. At our clinic, the Infectious disease department, we haven't noticed any other neurological syndromes associated with ongoing or recent hepatitis E. The possible explanation of a relatively higher incidence of meningitis in our patients compared to syndromes described in the literature could be the aim of our department. It is common to examine patients with headache, elevated body temperature or acute onset of hepatopathy at infectious disease departments in the Czech Republic, but not other neurological symptomatology without hepatopathy. We can therefore speculate that there were more cases of HEV-related neurological involvement in patients examined by neurologists during the reference period, but these patients were not diagnosed as having acute hepatitis E. The role of HEV in neurological disorders remains to be established. HEV is now recognized as a cause of peripheral nerve inflammatory disorders, notably acute inflammatory demyelinating polyneuropathy (AIDP) and neuralgic amyotrophy, with supposed pathophysiology of a variable blend of autoimmune phenomena. An association with central nervous system manifestations is likely, and the higher frequency of neurological disorders in immunocompetent patients suggests pathophysiological mechanisms involving the immune system [5].
Hepatitis E testing is therefore recommended in cases of neuralgic amyotrophy and Guillain-Barré syndrome, regardless of liver function tests [7]. This appears to be an appropriate next step in the differential diagnosis of serous meningoencephalitis after excluding more common CNS infections.
Funding
This study was funded by the Ministry of Health of the Czech Republic, grant number 17-31921A.
The Editorial Board declares that the manu script met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Matúš Mihalčin, MD
Department of Infectious Diseases
University Hospital Brno
Jihlavská 20
625 00 Brno
Czech Republic
e-mail: matus.mihalcin@gmail.com
Accepted for review: 26. 5. 2021
Accepted for print: 4. 11. 2021
An extended version of this article can be found at csnn.eu.
Zdroje
1. Státní zdravotní ústav. Výskyt vybraných infekcí v České republice hlášených v letech 2009–2018 na 100.000 obyvatel, SZÚ. [online]. Available from URL: http: //www.szu.cz/publikace/data/2018/vyskyt-vybranych-infekci-v-ceske-republice-hlasenych-v-1.
2. Izopet J, Tremeaux P, Marion O et al. Hepatitis E virus infections in Europe. J Clin Virol 2019; 120 : 20–26. doi: 10.1016/j.jcv.2019.09.004.
3. Dalton HR, Kamar N, van Eijk JJJ et al. Hepatitis E virus and neurological injury. Nat Rev Neurol 2016; 12 (2): 77–85. doi: 10.1038/nrneurol.2015.234.
4. Belbézier A, Lagrange E, Bouillet L. Neurologic disorders and hepatitis E: review of literature. Rev Med Interne 2018; 39 (11): 842–848. doi: 10.1016/j.revmed.2018.06.008.
5. Abravanel F, Pique J, Couturier E et al. Acute hepatitis E in French patients and neurological manifestations. J Infect 2018; 77 (3): 220–226. doi: 10.1016/j.jinf.2018. 06.007.
6. Fousekis FS, Mitselos IV, Christodoulou DK. Extrahepatic manifestations of hepatitis E virus: an overview. Clin Mol Hepatol 2020; 26 (1): 16–23. doi: 10.3350/ cmh.2019.0082.
7. European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol 2018; 68 (6): 1256–1271. doi: 10.1016/ j.jhep.2018.03.005.
Štítky
Dětská neurologie Neurochirurgie NeurologieČlánek vyšel v časopise
Česká a slovenská neurologie a neurochirurgie

2021 Číslo 6
-
Všechny články tohoto čísla
- Normotenzní hydrocefalus
- Synukleinopatie a jejich laboratorní biomarkery
- Diferenciální diagnostika glioblastomu a solitárních metastáz mozku – úspěch modelů umělé inteligence vytvořených na základě radiomických dat získaných automatickou segmentací z konvenčních MR sekvencí
- Klinicko-radiologický paradox u roztroušené sklerózy – význam vyšetření míchy
- Perorální kladribin v léčbě roztroušené sklerózy – data z celostátního registru ReMuS®
- Protein S 100B a jeho prognostické možnosti u kraniocerebrálních traumat
- Nodo-paranodopatie s protilátkami IgG4 proti neurofascinu-155
- Zobrazení průtoku mozkomíšního moku jednou sekvencí – variable flip angle turbo spin echo
- Aseptická meningitida při akutní hepatitidě E – zkušenosti z jednoho centra
- Rozsáhlé mnohočetné intraneurální gangliony peroneálního nervu
- Syndrom spinální sulkální arterie po stentem asistované embolizaci neprasklého aneuryzmatu vertebrální tepny embolizačním koilem
- Trombóza horní orbitální žíly
- Stiff -person syndrom
- ALBA and PICNIR tests used for simultaneous examination of two patients with dementia and their adult children
- Bilaterální paréza hlasivek v rámci recidivujících ischemických cévních mozkových příhod
- Guillain-Barrého syndrom u pacienta s COVID-19
- Obstrukční spánková apnoe u revmatoidního postižení subaxiální krční páteře
- Interpretace plazmatických hladin fenytoinu a valproátu při enterálním podávání u hypoalbuminemické pacientky
- Informace vedoucího redaktora
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle
- Stiff -person syndrom
- Normotenzní hydrocefalus
- Synukleinopatie a jejich laboratorní biomarkery
- Perorální kladribin v léčbě roztroušené sklerózy – data z celostátního registru ReMuS®
