Anti-HMGCR positive immune-mediated necrotizing myopathy
Authors:
J. Vejskal; P. Potužník
Authors place of work:
Neurologická klinika LF UK a FN Plzeň
Published in the journal:
Cesk Slov Neurol N 2023; 86(4): 279-281
Category:
Dopis redakci
doi:
https://doi.org/10.48095/cccsnn2023279
Dear Editor,
Immune-mediated necrotizing myopathies (IMNMs) are a subgroup of rare autoimmune myositis characterized by a typical histological picture from muscle biopsy, the presence of specific autoantibodies, a clinically proximal myopathic syndrome and response to immunosuppressive or immunomodulatory therapy. Within this group, we distinguish myositis associated with anti-SRP (anti-signal recognition particle) antibodies, anti-HMGCR (anti 3-hydroxy-3-methylglutaryl coenzyme A reductase) antibodies or necrotizing myositis seronegative (up to 20% of cases) [1].
The immune-mediated necrotizing myopathy associated with anti-HMGCR antibodies overlaps with a clinical entity called statin-induced necrotizing autoimmune myopathy (SINAM), which is based on the theory of induction of autoimmunity by hypolipidemic agents from the statin group [2-4].
The disease is rare with an incidence of two cases per million population per year [5], or two to three cases per 100,000 population treated with statins per year [6]. Clinically, it is characterized by proximal myopathic syndrome with or without muscle pain. Affection of other muscle groups (neck or swallowing muscles) is less common [5], and extra-muscular symptoms are rare [6]. The disorder progresses within weeks, often following statin use, with an interval of 2 months to several years from initiation or even cessation of treatment [5]. Female sex and age over 50 years are predominant, whereas younger age is less common and predicts a worse course of the disease and a poorer response to treatment; aetiologically, these cases are less frequently associated with statin use [7] and, rarely, may be paraneoplastic [8].
Laboratory findings include elevation of muscle enzymes with creatine kinase (CK) values in the order of ten times the normal [4]. On EMG we find myogenic findings with spontaneous denervation activity [4]. Muscle MRI demonstrates active inflammation in the early phase without a specific pattern of involvement of individual muscle groups [5], while also serving to target muscle biopsy [9]. In the histological picture we find necrosis of muscle fibres with minimal cellular inflammatory infiltration; overexpression of HLA I surface antigens with membrane-associated complement attack complex (MAC) is indicative of an ongoing autoimmune process [5,6]. Serologically, we demonstrate specific anti-HMGCR antibodies [5].
The mainstay of treatment is discontinuation of statins with their permanent contraindication followed by immunosuppressive therapy. In the first line, we choose corticosteroids in combination with corticoid-sparing immunosuppressants (azathioprine, methotrexate, mycophenolate mofetil or cyclophosphamide). Intravenous immunoglobulins (IVIG) or rituximab are available as rescue therapy. The therapeutic response to first-line treatment is usually good [5,6].
Between 2015 and 2023, a total of five patients with anti-HMGCR positive IMNM were diagnosed at our institution. We present here two cases.
A woman born in 1970, treated for type 2 diabetes mellitus, hypertension with hypercholesterolemia and depressive syndrome was examined in April 2018 for progressive pain and weakness of the upper and lower limb girdle muscles. Clinically, proximal myopathic syndrome was diagnosed, the patient was unable to raise her upper limbs above her head and had difficulty lifting herself out of a chair with the use of her arms. Laboratory findings were elevation of muscle enzymes (CK 101 μkat/l, myoglobin 1 140 μg/l). EMG initially with very mild myogenic findings.
Due to a history of statin use (atorvastatin 20 mg daily since April 2014), statin myopathy was thought and the drug was discontinued. She was hospitalized for follow-up due to persistent problems after discontinuation with persistent elevation of muscle enzymes.
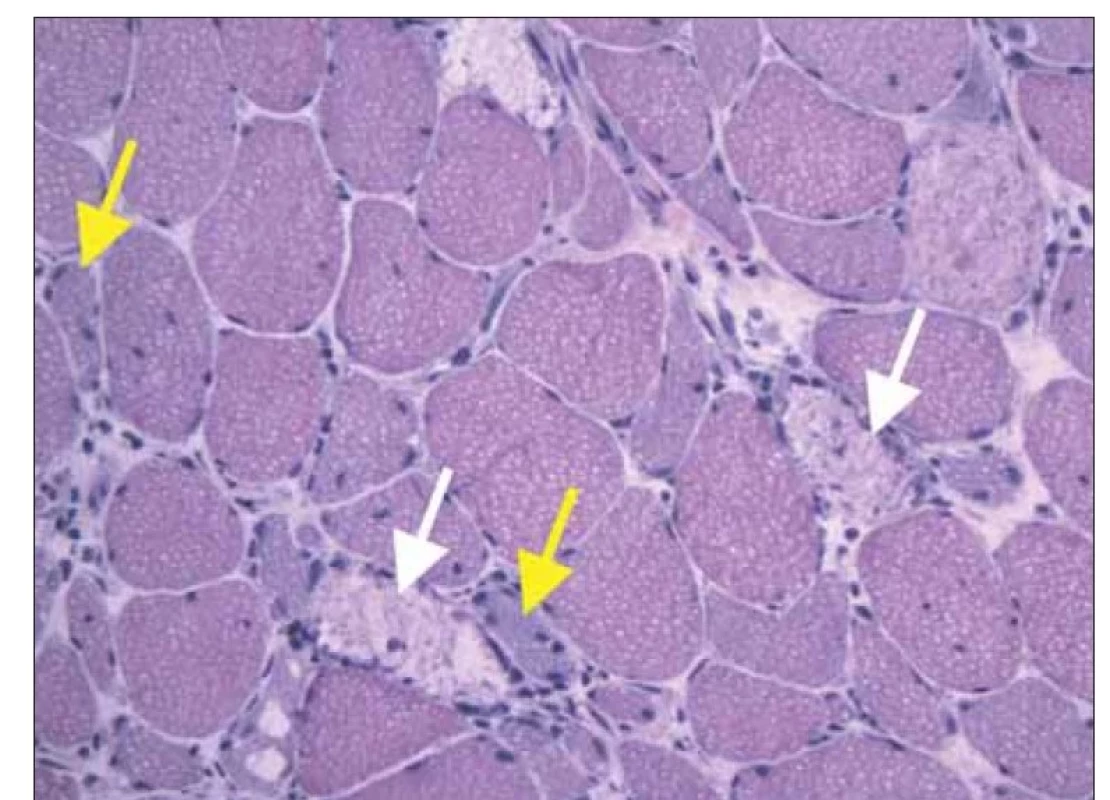
Clinically, a moderate myopathic syndrome was present with typical predilection for muscle weakness in the gidrles of the upper and lower limbs, with no involvement of the neck muscles, and no extra-muscular manifestations. EMG showed a myogenic lesion with spontaneous denervation activity. MRI of the proximal muscles of the lower limbs revealed oedema of the anterior thigh muscle group (Figure 1). Muscle biopsy of the m. vastus lateralis on the right was performed to confirm suspected diagnosis of inflammatory myopathy. Histological examination (Institute of Pathology and Molecular Medicine, Motol University Hospital in Prague) showed numerous muscle necroses, atrophy and regenerating myoblasts with minimal inflammatory infiltration (haematoxylin-eosin staining) (Fig. 2), as well as over-expression of HLA I molecules and numerous deposits of complement components on muscle fibre membranes (immunohistochemical imaging). Electron-microscopic imaging did not show any pathological findings. Histology was compatible with the diagnosis of IMNM. Examination of a panel of myositis-associated antibodies confirmed the positivity of anti-HMGCR antibodies (240.8 U/ml, Institute of Rheumatology, Prague).
Fig. 1. MRI of thighs, T2 turbo inversion recovery magnitude weighted sequence. There
is evident edema of mm. quadricipites bilateraly, dominantly in mm. recti femoris and
vasti femoris laterales (white arrows).
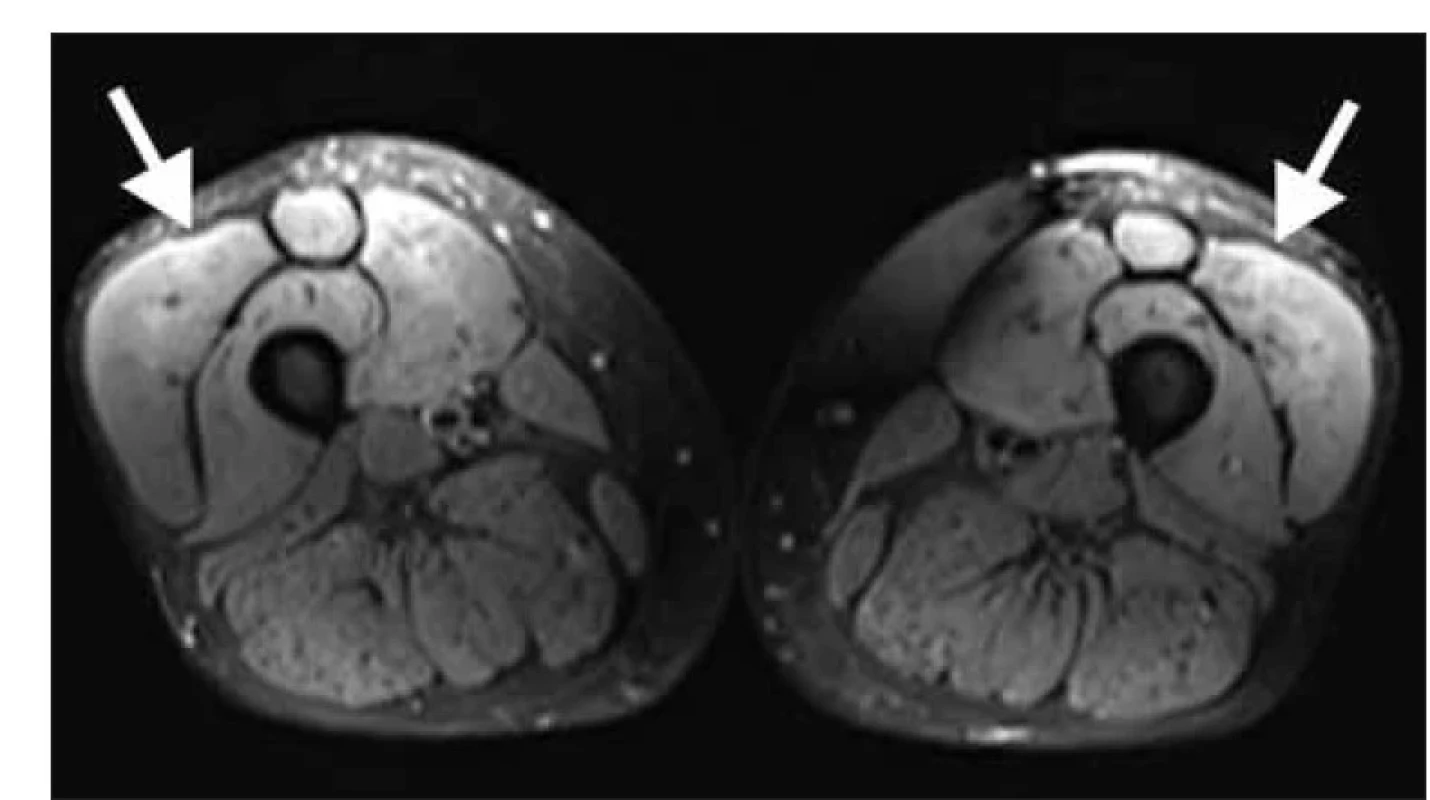
Fig. 2. Histology of muscle biopsy (rightside m. vastus lateralis), hematoxilin-eosin staining.
There are numerous necrotic muscle fibers (white arrows) with regenerating basofil myoblasts (yellow arrows). Only a few lymfocytes and macrophages are present as
a part of healing response.
Upon diagnosis of anti-HMGCR positive IMNM, we initiated immunosuppressive treatment, initially with an intravenous pulse of methylprednisolone followed by oral prednisone (1 mg/kg) in combination with azathioprine. The therapeutic effect was good. At the last follow-up in January 2023, muscle strength was found to be normal; anamnestically, the patient reported increased muscle fatigue after physical activity, and a slight elevation of muscle enzymes (CK 5.3 μkat/l, myoglobin 112 μg/l) persisted in the laboratory. Gradual de-escalation of corticotherapy is sought.
The second case - a woman born in 1992, of Vietnamese nationality, still completely healthy and without permanent medication - was examined in April 2022 for 9 months of progressive muscle weakness and pain with distribution in the upper and lower limb girdles and the neck area, anamnestic history of intermittent swallowing difficulties, without extra-muscular manifestations. Laboratory findings revealed elevation of muscle enzymes (CK 141 μkat/l, myoglobin 1 231 μg/l). EMG confirmed myopathic findings with spontaneous denervation. She was admitted for diagnostic hospitalization because of suspected myositis. MRI of thigh muscles revealed oedema with maximum involvement in the m. quadriceps. Biopsy of the m. rectus femoris muscle revealed a picture of necrotizing myopathy. Laboratory panel revealed anti-HMGCR autoantibody positivity (150.9 U/ml), but anamnestic history showed that the patient had never taken statins. We initiated immunosuppressive therapy, initially with an intravenous pulse of methylprednisolone, followed by a combination of prednisone (1 mg/kg) and azathioprine. Subjectively, the patient reported a slight improvement after 3 months of treatment; however, objective findings and elevation of muscle enzymes were stationary. In the absence of an effect of conventional treatment, we added whole-body PET/CT scanning, which ruled out a paraneoplastic aetiology, and after another 3 months we proceeded to administer intravenous immunoglobulins (2 g/kg). One month after treatment, there is a marked improvement in muscle strength and a decrease in muscle enzymes (CK 41 μkat/l, myoglobin 397 μg/l); monitoring of the treatment effect continues, and we are gradually de-escalating corticotherapy.
The case reports presented in our cohort show different forms of the disease. Even such a rare and laboratory and histologically narrowly specified myositis realistically manifests itself in a broader spectrum of clinical phenotypes. Four of our patients represent the "classic" form corresponding to the SINAM variant - i.e., a history of statin use (treatment initiation between 6 months and 4 years before the onset of myositis), older age (mean 63.5 years), myopathic presentation with limb girdle involvement only, and good response to immunosuppressive therapy. On the other hand, the last patient is young (30 years old), with no history of statin use, with a clinical picture including weakness of the neck and swallowing muscles, and does not respond to "first-line" treatment; race cannot be overlooked as an important factor. At the same time, a clear predominance of the female sex (100%) is confirmed in the cohort.
This is an unauthorised machine translation into English made using the DeepL Translate Pro translator. The editors do not guarantee that the content of the article corresponds fully to the original language version.
Zdroje
1. Allenbach Y, Benveniste O, Stenzel W et al. Immune-mediated necrotizing myopathy: clinical features and pathogenesis. Nat Rev Rheumatol 2020; 16 (12): 689–701. doi: 10.1038/s41584-020-00515-9.
2. Mohassel P, Mammen AL. Anti-HMGCR Myopathy. J Neuromuscul Dis 2018; 5 (1): 11–20. doi: 10.3233/JND-170282.
3. Needham M, Fabian V, Knezevic W et al. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul Disord 2007; 17 (2): 194–200. doi: 10.1016/j.nmd.2006.10.007.
4. Horák T, Voháňka S, Tvrdíková E et al. Statiny indukovaná nekrotizující autoimunitní myopatie. Cesk Slov Neurol N 2017; 80/113 (5): 569–577. doi: 10.14735/amcsnn2017569.
5. Babu S, Li Y. Statin induced necrotizing autoimmune myopathy. J Neurol Sci 2015; 351 (1–2): 13–17. doi: 10.1016/j.jns.2015.02.042.
6. Mammen AL. Statin-associated autoimmune myopathy. N Engl J Med 2016; 374 (7): 664–669. doi: 10.1056/ NEJMra1515161.
7. Milone M. Diagnosis and management of immune-mediated myopathies. Mayo Clin Proc 2017; 92 (5): 826–837. doi: 10.1016/j.mayocp.2016.12.025.
8. Mizuma A, Kouchi M, Netsu S et al. Paraneoplastic anti-3-hydroxy-3-methylglutary-coenzyme a reductase antibody-positive immune-mediated necrotizing myopathy in a patient with uterine cancer. Intern Med 2017; 56 (14): 1915–1918. doi: 10.2169/internalmedicine.56.8134.
9. Zámečník J. Svalová biopsie v deseti bodech. Cesk Slov Neurol N 2018; 81/114 (3): 358–361. doi: 10.14735/ amcsnn2018358
Štítky
Dětská neurologie Neurochirurgie NeurologieČlánek vyšel v časopise
Česká a slovenská neurologie a neurochirurgie

2023 Číslo 4
-
Všechny články tohoto čísla
- Editorial
- Diagnostika kořenové avulze u poranění brachiálního plexu před chirurgickým výkonem
- Klonální hematopoéza neurčitého potenciálu je možná a dosud nepopsaná příčina cévní mozkové příhody
- Zrakové evokované potenciály lze vyšetřit novým mobilním přístrojem kdekoliv
- Výsledky léčby aneuryzmatického subarachnoidálního krvácení u seniorů
- Psychometrická validácia dotazníka MSQOL-54 na Slovensku – pilotná štúdia
- Efekt dvojího úkolu na rychlost chůze u starších jedinců s kognitivním poklesem
- Neuralgická amyotrofie asociovaná s hepatitidou E jako vzácná příčina dyspnoe
- Anti-HMGCR pozitivní imunitně zprostředkovaná nekrotizující myopatie
- Manažment pacientov s roztrúsenou sklerózou liečených perorálnym kladribínom po štyroch rokoch od začiatku liečby
- Vyšetrenie ľahkého reťazca neurofilamentu v krvi pacientov s roztrúsenou sklerózou
- Kleinová L, Cerman J, Hlávka J et al. Nové farmakologické možnosti v léčbě Alzheimerovy nemoci.
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle
- Psychometrická validácia dotazníka MSQOL-54 na Slovensku – pilotná štúdia
- Diagnostika kořenové avulze u poranění brachiálního plexu před chirurgickým výkonem
- Klonální hematopoéza neurčitého potenciálu je možná a dosud nepopsaná příčina cévní mozkové příhody
- Anti-HMGCR pozitivní imunitně zprostředkovaná nekrotizující myopatie
