Results of surgical treatment of 15 patients with meralgia paresthetica
Výsledky chirurgické terapie meralgia paresthetica 15 pacientů
Cíl: Cílem této studie byla prezentace výsledků terapie syndromu meralgia paresthetica (MP) na našem pracovišti a souhrn současných chirurgických strategií v léčbě. Metodika: Diagnóza MP byla stanovena na základě kombinace typických klinických příznaků, negativního nálezu na MR bederní páteře, elektrofyziologických a ultrasonografických vyšetření nervus cutaneus femoris lateralis (lateral femoral cutaneous nerve; LFCN) a diagnostického nervového bloku. V případech selhání konzervativní terapie byla provedena chirurgická neurolýza LFCN. V případě přetrvávajících obtíží, byla pacientům nabídnuta chirurgická neurotomie. Výsledky: Patnáct pacientů (13 mužů, 2 ženy) bylo chirurgicky léčeno pro MP v letech 2006 -2020. Jedenáct případů mělo klasifikovanou idiopatickou a čtyři iatrogenní příčinu. Kromě typických bolestí, parestezií a dysestezie anterolaterální části stehna, mělo 13 pacientů také hypestezii v této oblasti. Průměrná předoperační hodnota bolesti hodnocená dle Visuální analogové škály (VAS) byla 8,4, která rok po chirurgické dekompresi klesla na průměr 2,2. Tři pacienti byli nespokojeni s výsledkem chirurgické neurolýzy (VAS nad 7), všichni měli iatrogenní příčinu MP. Z těchto tří pacientů jeden zvolil chirurgickou neurotomii LFCN. Závěr: Chirurgická neurolýza LFCN je efektivní terapie MP v případech, kdy konzervativní terapie selže. Neurotomie, i když jasně účinná, byla vyhrazena pro případy neúspěšné neurolýzy, protože je doprovázena trvalou hypestézií anterolaterálního stehna.
Klíčová slova:
neuropatie – meralgia paresthetica – neurolýza – neurotomie – dekomprese
Authors:
J. Lodin 1,2; Š. Brušáková 3; D. Kachlík 4; M. Sameš 1; I. Humhej 1,4
Authors‘ workplace:
Department of Neurosurgery, J. E. Purkyne University, Masaryk Hospital, Krajská Zdravotní a. s., Ústí nad, Labem, Czech Republic
1; Faculty of Medicine in Plzeň, Charles, University, Plzeň, Czech Republic
2; Department of Neurology, J. E. Purkyne University, Masaryk, Hospital, Krajská Zdravotní a. s., Ústí nad Labem, Czech Republic
3; Department of Anatomy, Second, Faculty of Medicine in Prague, Charles, University, Prague, Czech Republic
4
Published in:
Cesk Slov Neurol N 2022; 85(1): 38-43
Category:
Original Paper
doi:
https://doi.org/10.48095/cccsnn202238
Overview
Aim: The aim of this study was to portray results of meralgia paresthetica (MP) treatment at our institution, as well as a review of current surgical treatment strategies. Methods: Diagnosis of MP was made based on a combination of typical patient symptoms, negative MRI of the lumbar spine, electrophysiological and ultrasound examinations of the lateral femoral cutaneous nerve (LFCN) and a diagnostic nerve block. In cases where conservative therapy failed to improve the patient‘s symptoms, surgical neurolysis of LFCN was performed. In cases of unsatisfactory relief of clinical symptoms, surgical neurotomy was off ered to the patient. Results: Fifteen patients (13 males, 2 females) were surgically treated for MP from 2006 to 2020. Eleven cases were classified as idiopathic and four were classified as iatrogenic. In addition to typical pain, paresthesias and dysesthesias of the anterolateral thigh region, 13 patients presented also with hypesthesia of this region. Mean average preoperative Visual Analogue Pain Scale (VAS) was 8.4. After undergoing surgical decompression, the average postoperative VAS after one year was 2.2. Three patients were unsatisfied after surgical neurolysis (VAS over 7), all of whom had an iatrogenic cause of MP. Of these three patients, one opted for surgical neurotomy of the LFCN. Conclusion: Surgical neurolysis of the LFCN is an effective treatment for patients with MP in cases where conservative therapy fails. Neurotomy although clearly effective, was reserved for cases of failed neurolysis, as it is accompanied with permanent hypesthesia of the anterolateral thigh.
Keywords:
neuropathy – meralgia paresthetica – neurolysis – neurotomy – decompression
Introduction
Meralgia paresthetica (MP), also known as Bernhardt-Roth’s syndrome, is an entrapment neuropathy of the somatosensory lateral femoral cutaneous nerve (LFCN) firstly described by Hager in 1885 [1]. It is one of the most common mononeuropathies of the lower limb and is classically associated with pain, paresthesias, dysesthesias and sensory loss of the anterior and lateral aspect of the thigh [2,3]. Based on its suspected cause, it can be classified as idiopathic (associated with mechanical or metabolic factors) or iatrogenic (associated with surgical or interventional procedures within the proximity to the LFCN) [4]. However, diagnosis of MP is complex, and it is often mistaken for more common causes of proximal lower limb pain such as degenerative lumbar spine, sacroiliac joint or hip disorders [5]. As such, consideration of this disorder is crucial in the diff erential diagnosis of pathologies in this region. Once MP is diagnosed, various conservative and surgical treatment modalities are available including pressure alleviation of the nerve, kinesiotaping, nerve blocks, neurolysis or neurotomy [6].
We present our diagnostic approach, therapeutic strategy and long-term outcomes of fifteen patients who underwent surgical treatment of MP at our institution.
Methods
Diagnosis of MP
Patients were diagnosed with MP based on a combination of patient history, clinical examination, radiological examination, electrophysiological examination and a diagnostic nerve block. Firstly, a detailed patient history was obtained with special focus paid to prior surgical procedures, irradiation or trauma in the inguinal region, metabolic disorders and pain triggers (tight clothing, specific movements or activities, etc.). The disease was classified as iatrogenic if the patient underwent a surgical, interventional or radiation procedure in close proximity to the LFCN prior to developing symptoms of MP. All other cases were considered idiopathic and were further divided into mechanical or metabolic subgroups. Furthermore, patients were asked to describe their pain in detail, including its characteristics, intensity, distribution and triggers. Secondly, the patients underwent a clinical examination of the lower limbs with all sensory and motor deficits documented. Thirdly, an MRI of the lumbar vertebral column was performed to rule out a spondylogenic cause of the patients’ symptoms. A small number of patients also underwent a soft-tissue ultrasound of the LFCN; however, this examination was limited by patient obesity and was not required for MP diagnosis. The same was true for electrophysiological examination of the LFCN, which consisted of either conduction studies or somatosensory evoked potentials (SSEP). Finally, all patients underwent a diagnostic nerve block of the LFCN using 1% lidocaine or 1% bupivacaine. A positive result was considered if pain relief lasted for at least one hour after the block was performed. The diagnosis was confirmed in cases of typical clinical symptoms in combination with a negative lumbar vertebral column MRI. Positive diagnostic nerve block, EMG and ultrasound findings were supportive diagnostic modalities; however, they were not strictly required for the diagnosis.
Treatment options
In idiopathic mechanical cases of MP, all patients attempted conservative therapy including weight loss (if obesity was the suspected cause), oral analgesics, or alteration of clothing and physical activities. If conservative therapy failed, they were off ered surgical decompression of the LFCN. Patients in the iatrogenic and metabolic groups were primarily off ered surgical treatment along with patients whose attempted conservative therapy failed. In order to qualify for surgical treatment, patients were required to present with typical symptoms of pain, paresthesias, dysesthesias or sensory deficits limited to the anterior and lateral thigh region, to undergo a lumbar vertebral column MRI and a diagnostic nerve block. Surgical nerve decompression was then performed by a single surgeon using a standardized technique in global anesthesia. A linear skin incision was performed medial to the anterior superior iliac spine (ASIS) perpendicular to the inguinal groove (Fig. 1). The inguinal ligament was then identified at the ASIS and the LFCN was dissected medial to the ASIS at the origin of the sartorius muscle tendon under the fascia lata (Fig. 2). Decompression of the nerve was then performed proximally by partially incising inferior fibers of the overlying inguinal ligament and underlying iliac fascia, until the nerve was liberated up to its course on the anterior surface of the iliac muscle within the greater pelvis (Fig. 3). Distally, it was then inspected under the fascia lata down to its passage through the fascia approximately 5–6 cm distal to the ASIS up to its branches (Fig. 4). Afterwards, the patient was observed for 24 hours at the neurosurgical department and barring complications, released. The patient attended a follow-up visit at our outpatient clinic three months after surgery, where surgical eff ect was assessed based on a series of standardized questions. Parameters included pain intensity, residual paresthesias of the anterolateral thigh region and time period of pain remission. In cases where the patient did not have satisfactory pain relief, a second follow-up visit was scheduled three months later. A final examination was performed one year after the original surgery in all cases. If the patient continued to experience irritant symptoms, surgical neurotomy (neurectomy) was off ered as a salvage procedure. In these cases, we performed amputation of the common branch of the LFCN and inserted the proximal nerve stump deep into the greater pelvic region in order to avoid the presence of a painful amputation neuroma.
Obr. 1. Předoperační zobrazení operačního pole – spina iliaca anterior superior, linie
řezu kolmo k ligamentum inguinale (bílá šipka), plocha předního a laterálního stehna
inervovaná senzitivně nervus cutaneus femoris lateralis (bílá hvězda).

Obr. 2. Identifi kace nervus cutaneus femoris lateralis pod ligamentum inguinale. Ligamentum
inguinale (černá hvězda); nervus cutaneus femoris lateralis (černá šipka), útlak
nervus cutaneus femoris lateralis pod ligamentum inguinale (bílá šipka).

Obr. 3. Proximální dekomprese nervus cutaneus femoris lateralis částečným nastřižením
kaudální porce tříselného vazu. Ligamentum inguinale (černá hvězda), nervus cutaneus
femoris lateralis (černá šipka), útlak nervus cutaneus femoris lateralis pod inguinálním ligamentum
inguinale (bílá šipka).

Obr. 4. Kompletní dekomprese nervus cutaneus femoris lateralis proximálně k přední
ploše musculus iliacus. Ligamentum inguinale (černá hvězda), nervus cutaneus femoris
lateralis (černá šipka), uvolnění nervus cutaneus femoris lateralis pod ligamentum inguinale
směrem do velké pánve (bílá šipka).

Results
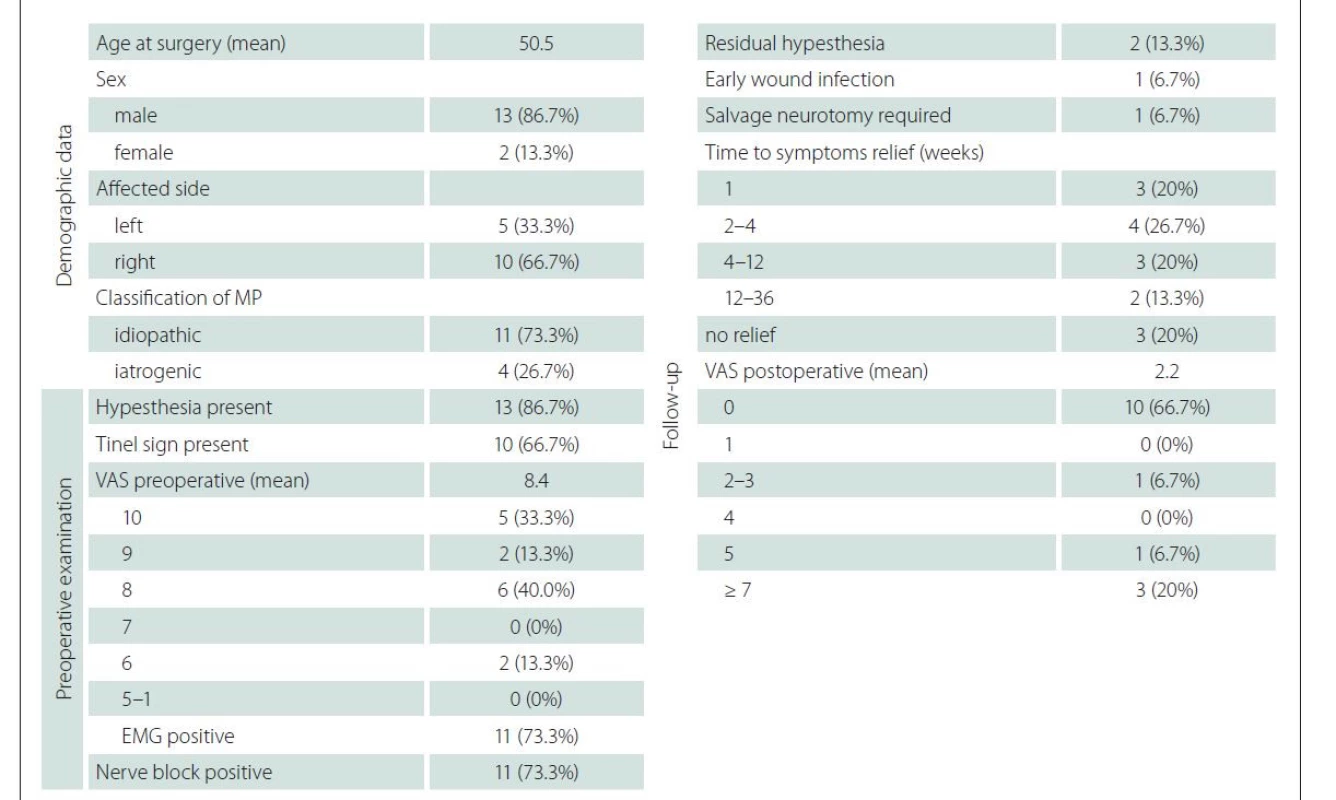
A total of fifteen patients were surgically treated for MP at our institution from 2006 to 2020 (Tab. 1). Thirteen were males and two were females with an average age of 50.5 (25–64) years. All patients presented with typical symptoms of MP as well as an MRI of the lumbar spine, negative for spondylotic causes of their symptoms. In addition to typical pain and dysesthesias, 13 patients presented with hypesthesia of the anterolateral thigh region and 10 had a positive Tinel sign medial to the ASIS. Their mean pain score was 8.4 based on the Visual Analogue Pain Scale (VAS), which significantly decreased after performing a diagnostic nerve block in 11 cases. Diagnostic EMG was performed in 11 cases, with positive findings consistent with MP and two patients underwent a diagnostic ultrasound of the inguinal region. Four cases of MP were identified as iatrogenic, following surgical procedures in the inguinal region (2 cases), interventional procedures utilizing the femoral artery (1 case) or radiation of the groin (1 case). The remaining 11 cases were classified as idiopathic mechanical with no metabolic cases present. All patients underwent surgical decompression of the LFCN without surgical complications. Postoperatively, one case was complicated by local infection, which resolved with the aid of antibio tic therapy within three weeks. In the follow-up period, the average VAS of all patients after one year was 2.2, with ten patients completely pain free, one patients relatively satisfied with a VAS below 3, one patient moderately satisfied with a VAS 5 and three patients unsatisfied with a VAS over 7. All three patients with unsatisfactory results after neurolysis had an iatrogenic cause of MP (previous radiation therapy – 1 case, previous surgery – 1 case, and previous interventional procedure – 1 case). Surgical neurotomy via alcoholization of the proximal nerve stump was offered to all three patients with one patient choosing to undergo this secondary procedure. Out of the twelve patients with a postoperative VAS below 5, three had residual postoperative hypesthesia.
Discussion
Management of patients presenting with MP is a complex issue. The entire process starts with correct diagnosis of this elusive entrapment neuropathy, which can be difficult even for an experienced specialist. In our experience, the most crucial step in diagnosing MP is obtaining a detailed patient history. In our patient series, all patients presented with typical pain, dysesthesias, paresthesias or hypesthesia in the anterolateral thigh region. Even so, all were required to undergo an MRI of the lumbar region to rule out L3 or L4 radiculopathy, which may present similarly to MP and are more common [7]. In order to specify the diagnosis of MP, we utilized two supportive diagnostic methods: diagnostic nerve blocks and electrophysiological studies. Several methods of local nerve blocks of the LFCN have been described as a diagnostic and in some cases even a therapeutic option for MP [8,9]. We utilized nerve blocks as a diagnostic tool, thus 1% bupivacaine solution without the presence of steroids was injected at the site just medial to ASIS. Although most studies advocate utilizing ultrasound to guide injections or even diagnose LFCN compression, we have found its use limited in obese patients, who represent an important part of our patient cohort [10]. In our patient series, four patients (27%) had negative results of the diagnostic nerve block; however, all had findings suggestive of MP on EMG. Electrophysiological studies performed at our institution consisted of antidromic nerve conduction studies of the LFCN based on the technique described by Oh et al [11], wherein electrodes are placed 4 cm medial to ASIS and 12 cm distally in a line connecting ASIS and the lateral portion of the patella. The conduction study must be performed bilaterally, as side comparison is commonly the most sensitive means of determining LFCN dysfunction [12]. Criteria for abnormality are shown in Tab. 2. In the case of obese patients, SSEP was utilized via stimulation of the lateral portion of the distal thigh and measured latency and amplitude of cortical response, again comparing both the left and the right LFCN. However, the sensitivity of SSEP is much lower than that in conduction studies [2]. In our series, 11 patients had findings indicative of MP on either conduction or SSEP studies. In the remaining four cases, it was not possible to obtain valid results due to obesity; however, these patients had a positive reaction to the diagnostic nerve block.

Once MP is diagnosed, two types of treatment modalities are available to patients: conservative (weight loss, oral analgesics, regime alterations) or surgical (neurolysis, neurotomy). Patients usually primarily attempt conservative treatment and if it is unsuccessful, opt for a surgical solution. Currently, literature regarding the superiority of neurolysis or neurotomy is inconclusive. The main advantage of neurolysis is preservation of LFCN integrity and thus its sensory function. Disadvantages include lower reported success rates compared to neurotomy, with a recent meta-analysis of 25 articles by Lu et al. demonstrating that 63% of patients were completely pain-free after neurolysis whereas 85% were pain-free after neurotomy [13]. As a consequence, revision surgery was required in 12% of patients after neurolysis, whereas none was required after neurotomy. Some authors suggest that reasons for neurolysis failure are associated with surgical technique, such as insufficient decompression, problems in identifying the LFCN or its anatomical variants. Aszmann et al have described the five most common variants in their study of 104 LFCNs and working knowledge of this variable anatomy is crucial prior to performing neurolysis of the LFCN [14]. Advantages of neurotomy include high success rates and a definitive resolution of the patient’s pain. The main drawback is anesthesia of the anterolateral thigh region, which is mostly well tolerated and may improve due to sprouting of neighboring sensory nerve axons. Unfortunately, the degree by which anesthesia of the anterolateral aspect of the thigh aff ects patient life quality has not been sufficiently investigated. One of the few studies evaluating the long-term eff ect of this complication was performed by de Ruiter et al, who showed that 62.5% of patients were not bothered, 25% were sometimes bothered and 12.5% were frequently bothered by numbness of the anterolateral aspect of the thigh [15].
In conclusion, the evidence supporting one surgical technique over another is lacking based on recent meta-analyses [15,16]. Our literature search yielded only one prospective study by de Ruiter et al, which demonstrated higher efficacy of neurotomy in pain relief (93.3%) compared to neurolysis (37.5%) [17]. However, their patient cohort comprised of only 22 patients and was not randomized or double-blinded, making its results susceptible to selection bias. In our patient series, 11 patients (73.3%) had satisfactory pain relief and one patient (6.7%) had moderate pain relief after neurolysis. This is in accordance with literary success rates, which range from 56 to 71% based on the meta-analysis of Lu et al [13]. Three patients (20%) had unsuccessful outcomes, and interestingly all had iatrogenic causes of MP. This suggests that the dominant cause of neuropathy was most likely the primary insult (mechanical or radiation damage), whereas compression of the nerve under the inguinal ligament was less significant. Neurotomy was then off ered to all three patients, and retrospectively, it is possible that it may have been a more suitable primary procedure. Nonetheless, only one patient chose to undergo surgery with the remaining two wary of a secondary procedure with possible long-term numbness of the anterolateral aspect of the thigh.
Conclusion
MP is an elusive clinical entity, which must be considered in patients with pain of the anterolateral aspect of the thigh with negative findings on lumbar spine MRI. In order to confirm the diagnosis, local anesthetic blocks or EMG may be performed as supportive diagnostic modalities. Treatment options include conservative and surgical approaches, with neurolysis and neurotomy being the most popular surgical modalities. Based on our results, we suggest neurolysis to be performed as a primary procedure in idiopathic cases of MP. Neurotomy should be reserved as a salvage procedure if neurolysis fails or in cases of iatrogenic MP as anesthesia of the anterolateral aspect of the thigh may be bothersome to some patients. Nonetheless, evidence for this therapeutic approach is currently lacking.
Ethical principles
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The study was performed in accordance with the Helsinki declaration from 1975 and its revisions (2004 and 2008). Patients signed informed consent with all diagnostic and therapeutic procedures, even when ethics committee consent is not required. The patients depicted in the accompanying figures consented to their publishing as long as they were anonymized.
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Ivan Humhej, MD, PhD
Department of Neurosurgery J. E. Purkyne University Masaryk Hospital, Krajská Zdravotní a.s. Sociální Péče 3316/12A 401 11 Ústí nad Labem Czech Republic
e-mail: ivan.humhej@kzcr.eu
Accepted for review: 23. 11. 2021
Accepted for print: 7. 2. 2022
Sources
1. Ivins GK. Meralgia paresthetica, the elusive diagnosis: clinical experience with 14 adult patients. Ann Surg 2000; 232(2): 281–286. doi: 10.1097/ 00000658 - 200008000-00019.
2. Seror P, Seror R. Meralgia paresthetica: clinical and electrophysiological diagnosis in 120 cases. Muscle Nerve 2006; 33(5): 650–654. doi: 10.1002/ mus.20507.
3. Sanjaya A. Meralgia paresthetica: finding an eff ective cure. Postgrad Med 2020; 132(1): 1–6. doi: 10.1080/ 00325481.2019.1673582.
4. Grossman MG, Ducey SA, Nadler SS et al. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg 2001; 9(5): 336–344. doi: 10.5435/ 00124635 - 200109000-00007.
5. Cheatham SW, Kolber MJ, Salamh PA. Meralgia paresthetica: a review of the literature. Int J Sports Phys Ther 2013; 8(6): 883–893.
6. Khalil N, Nicotra A, Rakowicz W. Treatment for meralgia paraesthetica. Cochrane Database Syst Rev 2012; 12(12): CD004159. doi: 10.1002/ 14651858.CD004 159.pub3.
7. Hirabayashi H, Takahashi J, Hashidate H et al. Characteristics of L3 nerve root radiculopathy. Surg Neurol 2009; 72(1): 36–40. doi: 10.1016/ j.surneu.2008.08.073.
8. Tagliafico AS, Torri L, Signori A. Treatment of meralgia paresthetica (Lateral Femoral Cutaneous Neuropathy): a meta-analysis of ultrasound-guided injection versus surgery. Eur J Radiol 2021; 139 : 109736. doi: 10.1016/ j. ejrad.2021.109736.
9. Tagliafico A, Serafini G, Lacelli F et al. Ultrasoundguided treatment of meralgia paresthetica (lateral femoral cutaneous neuropathy): technical description and results of treatment in 20 consecutive patients. J Ultrasound Med 2011; 30(10): 1341–1346. doi: 10.7863/ jum.2011.30.10.1341.
10. Palamar D, Misirlioglu TO, Arslan H et al. Adding an ultrasound scan before the injection reveals a rare cause of meralgia paresthetica: inguinal lymphadenomegaly. Am J Phys Med Rehabil 2020; 99(8): e93. doi: 10.1097/ PHM.0000000000001279.
11. Oh SJ. Clinical electromyography: nerve conduction studies. Philadelphia: Williams & Wilkins 2003.
12. Russo MJ, Firestone LB, Mandler RN et al. Nerve conduction studies of the lateral femoral cutaneous nerve. Implications in the diagnosis of meralgia paresthetica. Am J Electroneurodia gnostic Technol 2005; 45(3): 180 – 185.
13. Lu VM, Burks SS, Heath RN et al. Meralgia paresthetica treated by injection, decompression, and neurectomy: a systematic review and meta-analysis of pain and operative outcomes. J Neurosurg 2021; 1–11. doi: 10.3171/ 2020.7.JNS202191.
14. Aszmann OC, Dellon ES, Dellon AL. Anatomical course of the lateral femoral cutaneous nerve and its susceptibility to compression and injury. Plast Reconstr Surg 1997; 100(3): 600–604. doi: 10.1097/ 00006534 - 199709000-00008.
15. de Ruiter GC, Wurzer JA, Kloet A. Decision making in the surgical treatment of meralgia paresthetica: neurolysis versus neurectomy. Acta Neurochir (Wien) 2012; 154(10): 1765–1772. doi: 10.1007/ s00701-012-1431-0.
16. Payne R, Seaman S, Sieg E et al. Evaluating the evidence: is neurolysis or neurectomy a better treatment for meralgia paresthetica? Acta Neurochir (Wien) 2017; 159(5): 931–936. doi: 10.1007/ s00701-017-3136-x.
17. de Ruiter GC, Kloet A. Comparison of eff ectiveness of diff erent surgical treatments for meralgia paresthetica: results of a prospective observational study and protocol for a randomized controlled trial. Clin Neurol Neurosurg 2015; 134 : 7–11. doi: 10.1016/ j.clineuro.2015.04. 007.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2022 Issue 1
Most read in this issue
- Multiple tumefactive brain lesions as the first symptoms of demyelination
- Spontaneous intracranial hypotension
- Deep brain stimulation advances in neurological diseases
- Analytical and pre-analytical aspects of neurofilament light chain determination in biological fluids
