Preoperative Visual Memory Performance as a Predictive Factor of Cognitive Changes after Deep Brain Stimulation of Subthalamic Nucleus in Parkinson‘s Disease
Předoperační výkony v oblasti vizuální paměti jako prediktivní faktor kognitivních změn po hluboké mozkové stimulaci subthalamického jádra u Parkinsonovy nemoci
Cíl:
Hluboká mozková stimulace subthalamického jádra (DBS STN) je považována za klinicky přínosnou metodu léčby příznaků pokročilého stadia Parkinsonovy nemoci. Avšak i přes striktní dodržení vstupních kritérií může mít negativní dopad na kvalitu kognitivních funkcí. Cílem našeho výzkumu bylo nalézt prediktivní neuropsychologické faktory, které by již před DBS STN mohly signalizovat riziko zhoršení kognitivních funkcí po implantaci.
Soubor a metodika:
Výzkumný soubor byl tvořen 46 pacienty s idiopatickou Parkinsonovou nemocí (průměrný věk v době zákroku 59,61 let; SD = 7,06), kteří absolvovali neuropsychologické vyšetření před a po implantaci DBS STN. Neuropsychologická testová baterie zahrnovala zkrácenou verzi Wechslerovy intelektové škály, Mattisovu škálu demence, Seznam slov, Reyovu-Osterriethovu komplexní figuru, Stroopův test a zkoušky verbální fluence.
Výsledky:
Jako senzitivní prediktivní faktor ve vztahu k riziku kognitivních změn po implantaci DBS STN se prokázala kvalita vizuální paměti, kdy před implantací zjištěná úroveň vizuální paměti statisticky významně negativně korelovala s poklesem kognitivních výkonů v neuropsychologických testech po implantaci.
Závěr:
Nízké výkony v oblasti vizuální paměti před implantací mohou poukazovat na zvýšené riziko kognitivních změn po implantaci DBS STN u Parkinsonovy nemoci. Předpokládáme, že změny vizuální paměti odráží progresi degenerativního procesu u Parkinsonovy nemoci do dalších oblastí mozku mimo frontostriatální okruh, zejména do posteriorních temporoparietálních oblastí.
Klíčová slova:
hluboká mozková stimulace – subthalamické jádro – Parkinsonova nemoc – kognitivní změny – predikce kognitivních deficitů – vizuální paměť
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors:
Z. Hummelová 1,2; M. Baláž 1; E. Janoušová 3
Authors‘ workplace:
1stDepartment of Neurology, Faculty of Medicine of Masaryk University and St. Anne’s University Hospital in Brno
1; Department of Public Health, Faculty of Medicine of Masaryk University, Brno
2; Institute of Biostatistics and Analyses, Faculty of Medicine of Masaryk University, Brno
3
Published in:
Cesk Slov Neurol N 2016; 79/112(6): 680-686
Category:
Original Paper
doi:
https://doi.org/10.14735/amcsnn2016680
Overview
Aim:
Deep brain stimulation of subthalamic nucleus (DBS STN) is considered to be a clinically established treatment method to manage the symptoms of advanced stage Parkinson’s disease. Despite the strict inclusion criteria, it may have negative impact on the quality of cognitive functions. Our research aimed to identify predictive neuropsychological factors that signal risk of postoperative deterioration of cognitive functions before the DBS STN.
Patients and methods:
Forty-six patients with idiopathic Parkinson’s disease were included in the study (mean age at the time of operation 59.61 years; SD = 7.06). The patients were examined by a neuropsychologist before and after the DBS STN implantation. The neuropsychological test battery included Wechsler Adult Intelligence Scale short form, Mattis Dementia Rating Scale, Word list, Rey-Osterrieth Complex Figure Test, Stroop Colour Word Test and verbal fluency tests.
Results:
The quality of visual memory proved to be a sensitive predictive factor related to risk of cognitive changes after DBS STN implantation, as before the implantation the level of visual memory statistically negatively corresponded to impairment of cognitive performance in neuropsychological tests after the implantation.
Conclusion:
Low performance in the area of visual memory before the implantation may predict an increased risk of cognitive deterioration after the implantation of DBS STN in Parkinson’s disease. We assume that the changes in visual memory reflect progression of degenerative process in Parkinson’s disease into other brain areas away from the frontostriatal circuit, mainly to posterior temporoparietal areas.
Key words:
deep brain stimulation – subthalamic nucleus – Parkinson’s disease – cognitive changes – prediction of cognitive deficits – visual memory
Introduction
Deep brain stimulation (DBS) is currently considered to be a clinically beneficial treatment method for movement disorders, especially for Parkinson’s disease (PD). Stimulation of subthalamic nucleus (STN) is currently the most commonly used and probably the most effective treatment of the late motor complications of PD. A well-performed implantation of DBS STN should improve the patient’s quality of life, mainly related to the therapeutic effects it brings. It has been shown that cognitive problems and depression are factors with the most significant impact on the quality of life of patients with PD after DBS STN, even if there is an improvement in motor symptoms [1]. In order to obtain the best therapeutic effects of DBS STN, it is necessary to precisely consider inclusion and exclusion criteria when selecting suitable candidates (Tab. 1) [2,3] and to be aware of possible risk factors of cognitive decline after DBS STN in PD (Tab. 2) [2,4]. The tables show that the criteria mainly focus on neurological context. In relation to cognitive functions, mere presence of dementia or low cognitive performance are considered to be a risk factor. But what are the pre-operative neuropsychological risk factors of cognitive changes after DBS STN in PD? It should be noted that the specific topic of prediction of pre-operative cognitive deficits after DBS STN in PD has not yet been fully elucidated. Several publications deal with the standard evaluation of cognitive, behavioural and psychiatric changes after DBS STN in PD [1,4 – 8]. There only are sporadic publications focusing on cognitive aspects that increase the probability of cognitive deterioration after DBS STN in PD [4,9,10] with heterogeneous outcomes [4]. Some authors mention the role of quality of verbal memory (specifically learning a word list) and the score of total intelligence capacity [9], others point out significance of low pre-operative performance in the area of frontal functions or in the Initiation subtest of the Mattis Dementia Rating Scale [4], or significance of other pre-operative attention deficits [10]. Currently, there are no systematic studies in the Czech Republic searching for specific cognitive risk factors increasing the probability of cognitive deficit after DBS STN in PD.
![Inclusion and exclusion criteria for deep brain stimulation candidates with idiopathic PD [2,3].](https://www.csnn.eu/media/cache/resolve/media_object_image_small/media/image/51d45b78c320ea184a019d46188f2380.png)
![Risk factors of cognitive decline after deep brain stimulation of subthalamic nucleus for PD [2,4].](https://www.csnn.eu/media/cache/resolve/media_object_image_small/media/image/71337a64837abd91deb8f74d1f28ae5d.png)
Material and methods
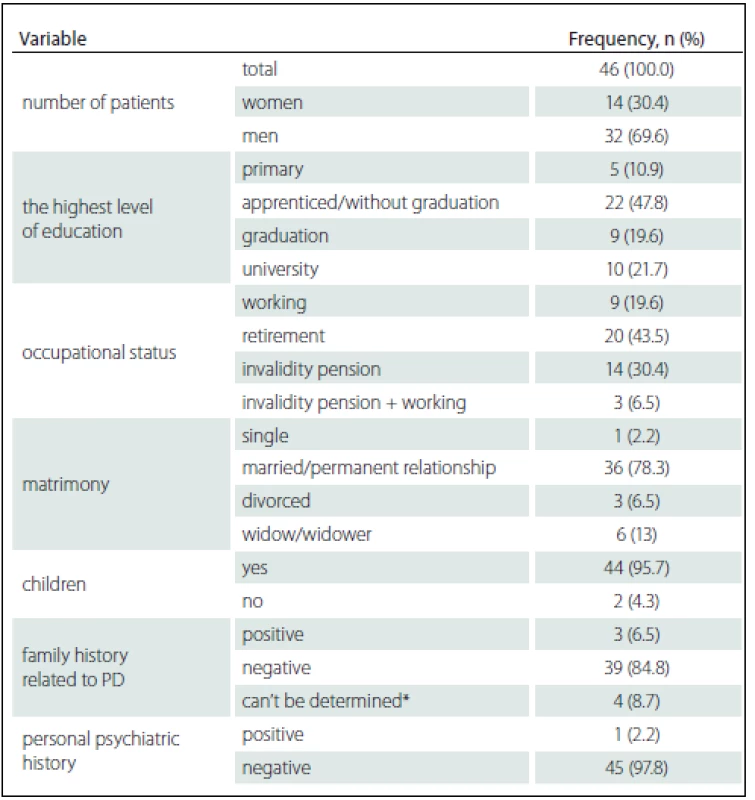
The aim of our research was to find out whether it is possible, with the use of pre-operation neuropsychological examination, to identify the cognitive characteristics that would help us to predict the risk of cognitive decline after DBS STN implantation in a group of patients with idiopathic PD included in the DBS STN implantation programme. The data were collected at the Movement Disorders Centre of the 1st Department of Neurology, St. Anne’s Faculty Hospital. The subject group included patients who fulfilled the inclusion and exclusion criteria listed in Tab. 1, who were able to undergo and complete neuropsychological examinations (including good graphomotor skills) so that the results could be statistically analysed, who were motivated for this type of treatment and were expected to benefit from it. The group comprised 46 patients, 14 women (30.4%) and 32 men (69.9%). The majority of included patients were men and this is because more men are affected by PD and more men than women are willing to undergo neurosurgery. Mean age at the disease onset was 49.11 years (SD = 7.09). Median disease stage was 2 according to the Hoehn and Yahr staging scale. Other descriptive, social-demographic and health characteristics of the research group are listed in Tab. 3. Neuropsychological examination was always performed before neurosurgery (M = 5.63; SD = 3.76 months) and then the patients were asked to under-go follow-up examination two years after the surgery (M = 23.46; SD = 7.07 months). The time lag eliminated the training effect. The neuropsychological examination included a shortened version of the Czech standardized version of the Wechsler Adult Intelligence Scale – Revised form (WAIS-R) [11,12], Mattis Dementia Rating Scale (DRS) [13], Word list (WL – from Wechsler Memory Scale III) [14], Rey-Osterrieth Complex Figure Test (ROCF) [15], Stroop Colour Word test [16], verbal fluency tests (VF – semantic and lexical) [17] and Montgomery Åsberg Depression Rating Scale (MADRS) [18]. The average level of pre-surgery intelligence capacity in the research group was at normal level (M = 103.72; SD = 11.46). None of the patients had signs of pre-surgery dementia, logically, some of them fulfilled the criteria for mild cognitive impairment (however, due to the total size of the research group we did not separate the mild impairment group). None of the patients manifested symptoms of specific impairments of symbolic functions during pre-surgical neuropsychological examination. One patient had a history of depressive disorder but none of the patients had signs of depressive disorder in the sense of diagnostic category before the surgery; the mean MADRS scale value M = 6.85 (SD = 4.15). The mean MADRS scale value after DBS STN, based on the follow-up neuropsychological examination after DBS STN was M = 9.17 (SD = 6.69). There was no statistically significant correlation between MADRS score and the cognitive performance for each cognitive test before and after DBS STN (Spearman’s Rank-Order Correlation) in our patient group. All patients in the research group were right-handed. The patients were examined during “on” state and full medication.
First, we statistically evaluated the differences in performance on neuropsychological tests before and after the DBS STN implantation. Subsequently, we sought to find out whether the quality of cognitive performances examined before the DBSSTN implantation correlated with statistically significant changes in cognitive neuropsychological tests after the DBS STN. Before this research phase, we tested whether there are any (positive or negative) statistically significant relationships between test performances before the surgery. We did not find any statistically significant negative correlations between cognitive performances before the operation. We found several statistically significant positive correlations between cognitive performances before the operation. This fact was taken into consideration when interpreting statistically significant correlations of cognitive changes after the implantation with cognitive performances before the implantation.
In our research, we did not work with a control group of patients. We therefore did not consider including a control group purposefully for the scientific evaluation of differences of cognitive performances in time. There are other studies that evaluated cognitive functions and behavioural changes after DBS STN implantation in PD with a similar design [6,9]. It was a crucial issue for us to compare neuropsychological performances before and after BDS STN and compare the development of cognitive skills in time.
The statistical software IBM SPSS Statistics 22 was used to process the acquired data. Demographic data and baseline characteristics were summarised using descriptive statistics. We did not expect normal data distribution due to the size and character of our research group. This assumption was verified with the Shapiro-Wilk test and, subsequently, non-parametric statistical tests were used to process the data. Comparison of performance differences on the different neuropsychological tests before and after DBS STN implantation was done using the Wilcoxon Signed Ranks Test. Spearman’s Rank-Order Correlation was used to measure relationships between variables. Multiple testing correction was not done due to the exploratory character of the analysis.
Results
Comparing the results of neuropsychological tests before and after DBS STN implantation, we identified more statistically significant changes of cognitive performances in the sense of their decline. However, from the clinical-psychological perspective, these changes were evaluated as mild; the differences in the mean performance values measured in the entire group before and after the implantation did not exceed the difference of – 1.5 SD for the given test score. As expected, there was performance decline in VF lexical (z = – 4.223; p < 0.001) – performance declined by more than – 1.5 SD in 12 (26%) patients – and in VF semantic (z = – 4.646; p < 0.001) – performance declined by more than – 1.5 SD in 11 (24%) patients. Statistically significant decline, but only in the clinically-psychological context, was also observed in the area of executive functions manifested in the Stroop test, part Words-Colours (z = – 3.135; p = 0.002) and Colours (z = – 4.423; p < 0.001), then in the Attention subtest of the DRS scale (z = – 3.107; p = 0.002) and in the DRS Initiation subtest (z = – 3.979; p < 0.001). On the contrary, no statistically significant performance decline was found in visual memory measured by the ROCF test or in the Construction subtest of the DRS scale. The differences in performance on neuropsychological tests are listed in Tab. 4.
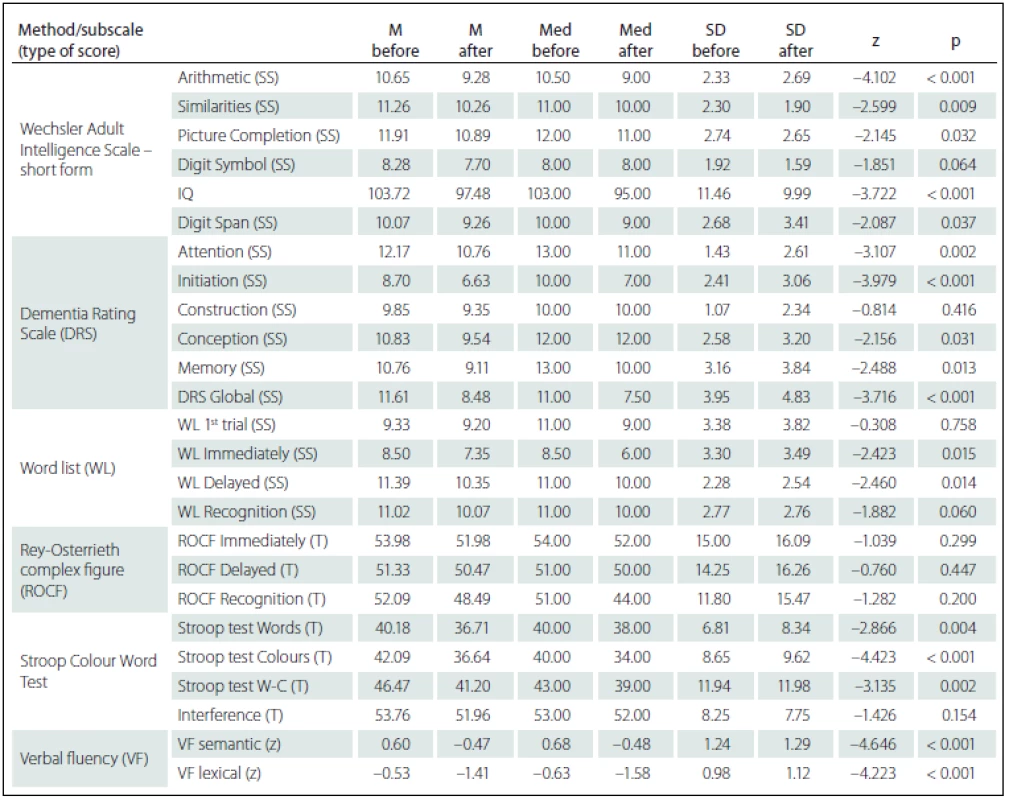
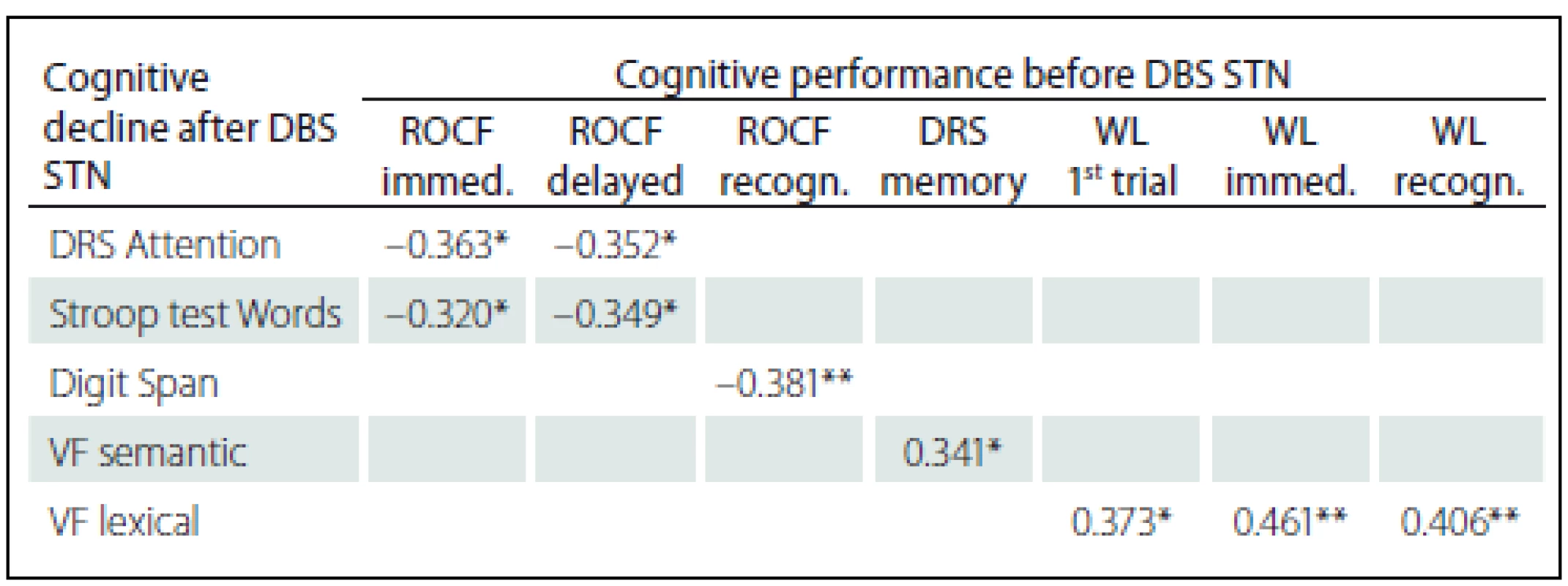
Statistically significant positive correlations in the results before and after DBS STN were found for VF lexical with pre-surgery performances in Word list test (before the surgery these tests did not show any statistically significant correlations); specifically, the difference between VF lexical and WL 1st attempt (rho = 0.373; p = 0.011), and Immediate WL (rho = 0.461; p = 0.001) and with Recognition WL (rho = 0.406; p = 0.005). We also found statistically significant negative correlations related to pre-surgery performances in the ROCF test, with a difference in cognitive test performances (before versus after DBS STN) in DRS Attention, Stroop test Words and Digit Span. Specifically, the difference in the DRS Attention showed negative correlation with the ROCF Immediate (rho = – 0.363; p = 0.014) and the ROCF Delayed (rho = – 0.352; p = 0.018). The difference in the Stroop test Words showed negative correlation with the ROCF Immediate (rho = – 0.320; p = 0.039) and the ROCF Delayed (rho = – 0.349; p = 0.023). The difference the Digit Span showed statistically significant negative correlation with the ROCF Recognition (rho = – 0.381; p = 0.010). Tab. 5 shows the results overview.
Discussion
In our research, we aimed to identify a cognitive functions indicator that could be used to predict risk of developing cognitive impairment after DBS STN implantation. In agreement with other studies, we proved mild negative impact of DBS on cognitive functions [7,19 – 21] that manifested in verbal fluency (semantic and lexical) [1,19,20,22] and in some aspects of executive functions (psychomotor speed performance), in the area of attention and working memory and in the area of strategic thinking [1,20]. Several changes in cognitive functions were statistically significant but from the clinical--psychological point of view we assessed these changes as mild.
The mechanism through which DBS STN subsequently leads to cognitive changes is not yet known [4] and is being discussed [22]. The key role of STN as part of basal ganglia is repeatedly described [4,5]. Similarly o striatum and pallidum, STN is divided into sensomotor, associative and limbic areas [5]. In particular, it is assumed that dorsolateral areas of STN are involved in motor functions, intermedial areas are important for cognitive processes and anteromedial areas are involved in emotions [4]. If an electrode is placed into motor areas of STN, we do not expect its direct effct on cognitive and emotional functions. However, since the electrode is placed in the miniature area of STN, it needs to be expected that the stimulation wll also afect surrunding areas togther with functional interference due to a small number of neurons overlapping in this anatomical structure [5]. Mainly the close functional connections with thalamus and frontal cortical areas of the brain and with frontostriatal circuit. Also, the mechanism of cognitive changes after DBS STN at a cellular level is unclear. One of the hypotheses is that while forced frequencies of STN activity improve motor functions, they may also interfere with phase activity of dopaminergic neurons associated with cognitive functions [21]. The profile of cognitive changes found in our research correlated with the above described neuroanatomical functional circuits and in neuropsychological context it may be summarized as mild frontostriatal deficit (verbal fluency, attention, information processing speed and verbal memory) [21].
Our research aimed to find the risk factors of cognitive changes after DBS STN. The ROCF test administered before the operation is one of the tests showing more correlations with decline in cognitive performances after DBS STN implantation; this test negatively correlated with post-surgical decline in the DRS Attention subtest, the Stroop tes Words and the Digit Span. Our statistical results lead to an assumption that poorer performance in ROCF tests before the operation is associated with greater decline in post-operative cognitive performance in the DRS Attention, Stroop test Words and Digit Span. All these tests measure, among other aspects, quality of attention and, in case of Digit Span, also working memory. What is the explanation of causal neuropsychological relations between these facts and which predictive information associated with cognitive risks after DBS STN imply, still remains to be answered.
In neuropsychological context, the ROCF test is a complex test integrating several cognitive processes. In Immediate and Delayed reproduction it measures mainly the quality of visual memory but good performance on this test also requires that attention processes and visuospatial functions are used. Despite partial decline in cognitive performance after DBS STN in our research group, it is the ROCF test that did not show any statistically significant performance decline after DBS STN in memory reproduction (both Immediate and Delayed). Therefore, it can be stated that in our research group visual memory was the ability that seemed stable in time, resistant to the effects of DBS STN or PD and, at the same time, correlated with cognitive changes related to frontostriatal circuit. It can be assumed that eventual pre-operative deficits in visual memory may be a significant signal for cognitive decline after DBS STN. The following knowledge and relations are of a great importance.
From neuroanatomical perspective, PD is predominantly associated with dysfunction of frontostriatal circuit due to impairment of neurotransmitter system, mainly dopamine deficit [6,24]. Consequently, patients with PD are expected to have changes in cognitive functions (mainly verbal fluency, executive and attention functions, spontaneous retrieval of memory information). However, for some aspects of memory process (encoding, retention, consolidation) medial temporal and hippocampal areas are crucial and their primary impairment during initial phases of cognitive changes related to PD are not usually expected. It has also been proven that Lewy bodies are relatively frequently found in PD, developing due to pathological accumulation of α-synuklein in subcortical and cortical brain areas [25]. It is also assumed that higher levels of Lewy bodies and more significant cholinergic depletion increase the risk of cognitive changes and accelerate development of dementia, mainly presenting with memory and visuospatial deficits. Some imaging studies performed in patients with early stages of PD found a relationship between cholinergic system and executive deficits and posteriorly located metabolic changes and multidomain cognitive disorder [26] – i.e. cognitive disorder related not exclusively to executive functions. Therefore, we can argue that frontal deficits in patients with PD are related to dopaminergic dysfunction, while cognitive deficits connected to posterior areas are associated with neuropathological changes in temporoparietal areas and were caused by pathological effects of Lewy bodies [25]. In other words, neuropathological correlates reflecting progression of neurodegeneration in PD include impairment of neuroanatomical regions critical for visual memory (i.e. ROCF) and for visuospatial processing. Therefore, we conclude that low performance in visual memory tests preoperatively may reflect the described progression of neurodegeneration out of the frontostriatal circuit into other brain areas.
Widely differing results were found in verbal memory as measured by the Word list. Statistically significant positive correlations between the difference in VF lexical performance after DBS STN implantation and preoperative performance in the WL test 1st attempt, WL Immediate and WL Recognition. Our results suggest that better perform-ance on the Word list test before implantation is associated with more significant decline in VF lexical. It has to be noted that statistically significantly poorer performance before DBS STN was observed in both the Word list test (WL Immediate, WL Delayed) and the VF lexical. We are inclined to an interpretation that this apparent illogicality may directly reflect neuroanatomical dissociation of the respective cognitive abilities. Crucial neuroanatomical relation with frontal areas and circuits is expected for VF lexical [27]. On contrary, the ability to learn (WL Immediate) and quality recognition of memory material (WL Recognition) means that the memory material is already engraved and stored (i.e. medial temporal and hippocampal components are intact).
It is of note that, according to our results, executive functions did not appear to indicate possible postoperative cognitive deficits. Probably because deficits in executive functions are characteristic and somehow ever-present in patients with PD and, therefore, their presence before and their decline after DBS STN can be expected [4,10,26]. This conclusion is in agreement with another study focusing on search for cognitive risk factors related to DBS STN in IPD [10].
Conclusion
In our research, the quality of visual memory evaluated by the ROCF test was proven to be a sensitive predictive factor of cognitive decline after DBS STN. Specifically, poor performance in visual memory before implantation may suggest higher risk of cognitive decline after implantation. From the neuroanatomical perspective, we assume that the quality of visual memory reflects progression of degenerative process in PD into other brain areas away from frontostriatal citcuit, mainly into posterior temporoparietal areas.
It is known that cognitive changes after DBS STN are one of the factors that fundamentally influence perception of quality of life by patients with PD after DBS STN. In our opinion, prediction of cognitive deficits is an important part of care of patients with PD, including the DBS STN therapy. This issue is currently being researched and we believe that our findings contribute relevant evidence for its further development.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Zuzana Hummelová M.A.
1st Department of Neurology
Faculty of Medicine of Masaryk University
St. Anne’s University Hospital in Brno
Pekařská 53
656 91 Brno
e-mail: zuzana.fanfrdlova@fnusa.cz
Accepted for review: 11. 11. 2015
Accepted for print: 4. 3. 2016
Sources
1. Witt K, Daniels Ch, Reiff J, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomized multicentre study. Lancet Neurol 2008;7(7):605 – 14. doi: 10.1016/ S1474-4422(08)70114-5.
2. Baláž M, Rektor I. Neuropsychologické a kognitivní vlivy hluboké mozkové stimulace subthalamického jádra u pacientů s Parkinsonovou nemocí. Neurol Praxi 2008;9(5):305 – 7.
3. Benabid AL, Chabarde S, Mitrofanis J, et al. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol 2009;8(1):67 – 81. doi: 10.1016/ S1474-4422(08)70291-6.
4. Massano J, Garrett C. Deep brain stimulation and cognitive decline in Parkinson’s disease: a clinical review. Front Neurol 2012;3 : 66. doi: 10.3389/ fneur.2012.00066.
5. Castrioto A, Lhommeé E, Moro E, et al. Mood and behavioural effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol 2014;13(3):287 – 305. doi: 10.1016/ S1474-4422(13)70294-1.
6. Kim HJ, Jeon BS, Paek SH, et al. Long-term cognitive outcome of bilateral subthalamic deep brain stimulation in Parkinson’s disease. J Neurol 2014;261(6):1090 – 6. doi: 10.1007/ s00415-014-7321-z.
7. Halpern CH, Rick JH, Danish SF, et al. Cognition following bilateral deep brain stimulation surgery of the subthalamic nucleus for Parkinson’s disease. Int J Geriatr Psychiatry 2009;24(5):443 – 51. doi: 10.1002/ gps.2149.
8. Smeding HM, Speelman JD, Koning-Haanstra M, et al. Neuropsychological effects of bilateral STN stimulation in Parkinson’s disease: a controlled study. Neurology 2006;66(12):1830 – 6.
9. Yágüez L, Costello A, Moriarty J, et al. Cognitive predictors of cognitive change following bilateral subthalamic nucleus deep brain stimulation in Parkinson’s disease. J Clin Neurosci 2014;21(3):445 – 50. doi: 10.1016/ j.jocn.2013.06.005.
10. Abbound H, Floden D, Thompson NR, et al. Impact of mild cognitive impairment on outcome following deep brain stimulation. Summary for Parkinson’s disease. Parkinsonism Relat Disord 2015;21(3):249 – 53. doi: 10.1016/ j.parkreldis.2014.12.018.
11. Říčan P, Šebek M, Vágnerová M. WAIS-R – Wechslerův inteligenční test pro dospělé – česká adaptace. Bratislava: Psychodiagnostické a didaktické testy 1983.
12. Randolph Ch, Mohr E, Chase TN. Assessment of intellectual function in dementing disorders: validity of WAIS-R short forms for patients with Alzheimer’s, Huntington’s and Parkinson’s disease. J Clin Exp Neuropsychol 1993;15(5):743 – 53.
13. Pedraza O, Lucas JA, Smith GE, et al. Robust andExpanded Norms for the Dementia Rating Scale. ArchClin Neuropsychol 2010;25(5):347 – 58. doi: 10.1093/ arclin/ acq030.
14. Wechsler D. Wechslerova paměťová škála. 3. vyd. Česká experimentální verze. Brno: Psychodiagnostika s.r.o. 1999.
15. Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial. Professional Manual. Odessa, Fl: Psychological Assessment Resources, Inc. 1995.
16. Golden JC, Freshwater SM. Stroop Color and Word Test. Adult Version. A Manual for Clinical and Experimental Uses. Wood Dale, IL: Stoelting Co 2002.
17. Preiss M, Bartoš A, Čermáková R, et al. Neuropsychologická baterie Psychiatrického centra Praha. 3. vyd. Praha: Psychiatrické centrum Praha 2012.
18. Leentjens AF, Verhey FR, Lousberg R, et al. The validity of the Hamilton and Montgomery-Asberg depression rating scales as screening and diagnostic tools for depression in Parkinson’s disease. Int J Geriatr Psychiatry 2000;15(7):644 – 9.
19. Castelli L, Perozzo P, Zibetti M, et al. Chronic deep brain stimulation of the subthalamicus nucleus for Parkinson’s disease: effects on cognition, mood, anxiety and personality traits. Eur Neurol 2006;55(3):136 – 44.
20. Moretti R, Torre P, Antonello M, et al. Neuropsychological changes after subthalamic nucleus stimulation: a 12 month follow-up in nine patients with Parkinson’s disease. Parkinsonism Relat Disord 2003;10(2):73 – 9.
21. Telecká S, Baláž M, Rektorová I, et al. Jeden rok po hluboké mozkové stimulaci pacientů s Parkinsonovou nemocí – neuropsychologické výsledky. Cesk Slov Neurol N 2010;73/ 106(1):57 – 61.
22. Denheyer M, Kiss ZH, Haffender AM. Behavioural effects of subthalamic deep brain stimulation in Parkinson’s disease. Neuropsychologia 2009;47(14):3203 – 9. doi: 10.1016/ j.neuropsychologia.2009.07.022.
23. Azulay JP, Witjans T, Eusebio A. Effect of subthalamic deep brain stimulation on non-motor fluctuations in Parkinson’s disease. J Neural Transm 2013;120(4):655 – 7. doi: 10.1007/ s00702-012-0958-9.
24. Ray N, Strafella AP. Dopamine, reward, and frontostriatal circuitry in impulse control disorders in Parkinson’s disease: insight from functional imaging. Clin EEG Neurosci 2010;41(2):87 – 93.
25. Svenningsson P, Westmann E, Aarsland D. Cognitive impairment in patiens with Parkinson’s disease: diagnosis, biomarkers, and treatment. Review. Lancet Neurol 2012;11(8):697 – 707. doi: 10.1016/ S1474-4422(12)70152-7.
26. McKinlay A, Grace RC, Darlymple-Alford JC, et al. Characterics of executive function impairment in Parkinson’s disease patients without dementia. J Int Neuropsychol Soc 2010;16(2):268 – 77. doi: 10.1017/ S13556177099 91299.
27. Hummelová Z, Janoušová E. Limity zkoušky verbální fluence v diferenciální diagnostice neurologických onemocnění. Cesk Slov Neurol N 2014;77/ 110(4):487 – 92.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2016 Issue 6
-
All articles in this issue
- Depression in Selected Neurological Disorders
- Proposed MRI Safety Monitoring of Patients with Multiple Sclerosis Treated with Natalizumab
- Do not Test but POBAV (ENTERTAIN) – Written Intentional Nam ing of Pictures and their Recall as a Brief Cognitive Test
- Executive Function Deficits in Patients with Blepharospasm
- The Importance of Thermal Threshold Testing in Detecting of Small Fiber Neuropathy in Type 1 Diabetes Mellitus
- A Case of Severe Progres sion of HIV-1 Meningoencephalitis and Lues Secundaria
- Autoimmune Encephalitis – Case Reports
- Anterior Cervical Osteophytes Causing Dysphagia and Dyspnea – Two Case Reports
- Pain-related Fear in Chronic Low Back Pain Patients
- The Use of Transcranial Sonography at Neuro-psychiatry Interface
- Introduction to Neuromuscular Ultrasound
- Surgical Treatment of Extensive Fibrous Dysplasia in the Craniofacial Region – a Case Report
- Preoperative Visual Memory Performance as a Predictive Factor of Cognitive Changes after Deep Brain Stimulation of Subthalamic Nucleus in Parkinson‘s Disease
- Orbital Cellulitis as a Complication of Acute Rhinosinusitis – our Experience with Treatment in Adult Patients
- Spinal Gossypiboma 20 years after Lumbar Discectomy – a Case Report
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Anterior Cervical Osteophytes Causing Dysphagia and Dyspnea – Two Case Reports
- Depression in Selected Neurological Disorders
- Autoimmune Encephalitis – Case Reports
- Surgical Treatment of Extensive Fibrous Dysplasia in the Craniofacial Region – a Case Report
