Dietary approaches specific to patients with multiple sclerosis
Authors:
K. Vodehnalová; E. Kubala; Havrdová; D. Horáková
Authors‘ workplace:
Neurologická klinika a Centrum klinických neurověd 1. LF UK a VFN v Praze
Published in:
Cesk Slov Neurol N 2023; 86(1): 25-30
Category:
Review Article
doi:
https://doi.org/10.48095/cccsnn202325
Overview
Interest in non-pharmacological treatment of MS is considerable among doctors and patients. In the light of new scientific findings, dietary intervention appears to be a promising adjunctive treatment to established pharmacotherapy. In patients with MS, the diet can lead to suppression of autoimmune inflammation, neurodegeneration and support of remyelination. Several possible specific nutritional directions are now being discussed in professional and patient circles. Thus, it is necessary to focus on the evidence behind them, so that the physician can report to the patient the possible benefits and risks associated with them. At first glance, different dietary approaches in the form of a low-fat or high-fat diet may have similar benefits on the course of MS. However, in the overall approach to the diet of patients with MS, we should continue to adhere to the rules of a rational diet with an emphasis on a high intake of vegetables, fruits and healthy fats.
Keywords:
Diet – Multiple sclerosis – Swank – Wahls – ketogenic
This is an unauthorised machine translation into English made using the DeepL Translate Pro translator. The editors do not guarantee that the content of the article corresponds fully to the original language version.
Introduction
Multiple sclerosis is an inflammatory and neurodegenerative demyelinating disease of the CNS caused by an autoimmune reaction. The origin of the disease is multifactorial, there is a combination of genetic predisposition of the individual with the influence of the environmental environment. The term environmental here includes in particular the influence of previous infections, vitamin D levels, environmental cleanliness and our lifestyle.
A pioneer in the study of the influence of food intake on MS was Canadian physician Roy L. Swank, who in 1948 presented his theory of the harmful effects of the Western diet on the course of the disease. That same year, he began recruiting patients for a study to confirm this idea. Attention to the effect of dietary approaches on MS has been increasing since 2000, when the number of published peer-reviewed studies began to exceed a dozen articles per year. The gradual acceleration of interest in the topic has so far culminated in 2021, when, according to the PubMed database, 1,119 peer-reviewed scientific papers were published as of July 10, 2022, after entering the keywords "multiple sclerosis" and "diet". Scientific analyses have been conducted on complex dietary styles, but also on the effects of individual foods or food components on the course of MS. In real life, it is very difficult to conduct a methodologically sound study that could unequivocally lead to the conclusion that the change in a patient's condition was caused by diet alone, as there are always other environmental factors (smoking, exercise, vitamin D intake, etc.) or drug therapy that undoubtedly influence the course of the disease. Aware of this research pitfall, it is not surprising that almost every recommendation is contradicted. Many dietary and nutritional questionnaires do not distinguish substantial differences in the quality of food intake. Thus, if researchers only collect information on the proportions of individual nutrients (fats, carbohydrates, proteins) but do not look more closely at the quality and process of food preparation, we may learn, for example, that a diet based on fat can be both beneficial and harmful. The importance of diet quality in MS patients has been investigated by two large studies (Health Outcomes and Lifestyle In a Sample of people with Multiple Sclerosis [HOLISM], The North American Research Committee on Multiple Sclerosis [NARCOMS]), which, when evaluated, came to the same conclusion that patients with the highest diet quality had a 20-30% lower chance of adverse disease course [1,2]. The aim of this summary article is to focus on the possible mechanisms by which diet influences disease progression and to list the most common special dietary approaches followed by patients. For a clearer message, we will focus only on recommendations that may favorably influence the course of MS and their biochemical rationale. The interest in the non-pharmacological approach among patients is high, according to a questionnaire survey almost 65% of patients have tried such an approach [3].
Mechanisms of action of diet
The pathogenesis of MS is based on an autoimmune inflammatory process in the CNS that causes acceleration of neurodegeneration. Influencing inflammation and neurodegeneration are the main pharmacological therapeutic targets. Another therapeutic target could be the remyelination of damaged pathways [4]. According to known facts, the effect of diet on all three modalities can be traced. However, the most studied component of MS pathogenesis, especially in relation to the development of new drugs, is inflammation. This is most likely due to the fact that the animal model of MS (Experimental Autoimmune Encephalomyelitis; EAE) is a model of mainly autoimmune inflammation, less able to simulate in patients the naturally accompanying neurodegeneration [5]. However, we can trace nutritional recommendations to influence all three therapeutic targets from scientific studies.
Influence of inflammation
The pathogenesis of the inflammatory component of MS is due to the response of the peripheral immune system and the inflammation itself in the CNS. Secondarily, the composition of the gut microbiome also influences the course of the disease. Influencing all three components can be achieved through diet [6].
The peripheral immune system, especially T-lymphocyte differentiation, is influenced by diet through suppression of the major pro-inflammatory transcription factors (NK-k B and AP-1). A diet rich in polyphenols (e.g. broccoli, blackcurrants, plums), sulphur (e.g. onions, garlic), omega-3 fatty acids (e.g. fish oil), butyrate (produced by the gut microbiome after consumption of fibre-rich foods), lycopene (e.g. tomatoes), carotenoids (e.g. carrots), lipoic acid (e.g. red meat, tripe, spinach), isothiocyanates (cruciferous vegetables), tocopherol (e.g. milk, nuts) [7].
Oxidative stress and the production of reactive oxygen radicals (ROS), which are generated by macrophages and microglia, play a major role in CNS inflammation. ROS damage the cell membrane of neurons and can lead to their death by necrosis or apoptosis [8]. Promising antioxidants in MS include vitamin C, vitamin E, lipoic acid or ketone bodies. While vitamin replacement had a significant effect on the course of MS only in animal models, regular lipoic acid replacement over 2 years resulted in a reduction of cerebral atrophy, an increase in walking speed, and a subjective decrease in fatigue and depression in patients with secondary progressive MS [9-11]. Ketone bodies not only act as antioxidants themselves, but also increase the levels of another antioxidant, glutathione [12].
Recently, there has been a growing interest in the influence of the gut microbiome on the onset and course of MS. In MS patients, an increased proportion of unfavourable bacteria, dysbiosis (e.g. Archaea, E. coli, Clostridium), has been found to indirectly affect the immune balance between Treg and Th17 lymphocytes through its metabolites, and thus may trigger or potentiate autoimmune reactions [13,14]. Diet has an absolutely crucial influence on the quality of the microbiome. The so-called Western diet, hypercaloric, with high intake of both sugars and fats at the same time, with industrially produced additives and preservatives, has been evaluated as the most harmful, and even a single day on this diet is enough to affect the gut microbiota [15]. Positive influence on the composition of the gut microbiota through diet is based, for example, on regular intake of fibre, omega-3 fatty acids and vitamin D3 [16]. Certain gut bacteria ferment fiber into short chain fatty acids (SCFAs), which support the integrity of the intestinal wall and promote the differentiation of anti-inflammatory Treg lymphocytes [17]. The intermittent fasting method in EAE achieved enrichment of the gut microbiota with beneficial bacteria (from the genera Bacteroidaceae, Lactobacillaceae, Prevotelaceae), whose rise correlated with a decrease in blood levels of pro-inflammatory leptin. Furthermore, through diet-induced ketone bodies and enhanced glutathione metabolism, the antioxidant response of the organism was increased. Reductions in the pro-inflammatory cytokine IL-17 and an increase in Treg in the intestinal wall were also observed [18].
Influence of neurodegeneration
According to current knowledge, inflammation in the CNS is responsible for the faster neurodegeneration in MS patients. Using MRI, we can measure the annual brain volume loss, which is around 0.2-0.5% per year in healthy adults [19], whereas in MS patients the range of loss is between 0.22-2.1% per year [20]. In inflammation, oxidative stress causes damage to mitochondria, leading to redistribution of ion channels, their insufficient function and consequently to the death of oligodendrocytes or neurons, i.e. neurodegeneration [21]. A higher intake of antioxidants could also contribute to reducing the effect of oxidative stress. One of the mechanisms of action of antioxidants, such as flavonoids or phytopigments from fruits and vegetables, could be through activation of AhR (aryl hydrocarbon receptor), which inhibits the activation of monocytes and microglia, which are also responsible for neurodegeneration [22,23]. Improvement in symptoms of other neurodegenerative diseases and EAE has been shown with regular intake of blueberries, strawberries or spinach [24,25].
Oligodendrocytes responsible for axon myelination are highly sensitive to energy deprivation [26]. When sufficient energy supply is provided to mitochondria, both oligodendrocyte and neuronal survival is improved. Inadequate energy supply to neurons during neurodegeneration may also be due to glucose hypometabolism, where there is a progressive reduction in the use of available glucose by the neuron, leading to mitochondrial dysfunction and subsequent apoptosis. These changes have been observed in neurons even before the clinical manifestations of neurodegeneration [10]. A solution to this paradoxical situation, where the body tries to provide neurons with enough fuel to produce energy through glucose in the bloodstream, but which the neurons cannot use and hence are starved, could be the introduction of a ketogenic diet. Ketone bodies are known to be an alternative but quite sufficient energy substrate for neurons. Ketogenic hypometabolism has not yet been described [27]. In one study, it was shown that 40% of brain tissue in MS patients had lower glucose utilization than healthy controls [28].
Influence of remyelination
Remyelination as a natural process of axon repair is crucial for the resolution of seizure symptoms in MS patients. Remyelination is mediated by oligodendrocyte precursor cells (oligomeric proanthocyanidins; OPCs), which are significantly less abundant in the CNS in the chronic form of MS than in the relapsing-remitting form [29]. An increase in OPCs occurs with dietary intervention in the form of a fasting-mimicking diet (FDM). Furthermore, this diet results in an increase in remyelination-promoting brain-derived neurotrophic factor (BDNF) [30].
Specific diets in MS
The effect on the course of the disease has been studied for individual foods, nutritional supplements and diets. Below we focus on the most researched dietary guidelines in MS.
Mediterranean diet
The Mediterranean diet is harder to define. It is a high intake of unsaturated fats, vegetables, fruit, nuts and whole grains, with a reasonable intake of fish, poultry and wine, and a limited intake of dairy products, red meat and sweets. The exact mechanism of the beneficial effect of this diet is not known, but it is likely to benefit from the rich intake of beneficial food components (omega-3 fatty acids, polyphenols, flavonoids, etc.) whose antioxidant and anti-inflammatory effect on the CNS has been mentioned above. The diet is known to prevent cardiovascular disease and age-related cognitive deficits, incl. Alzheimer's disease [31]. Reduced fatigue, narrower waist circumference and lower rates of overall disability have been demonstrated in MS patients on this diet [32,33].
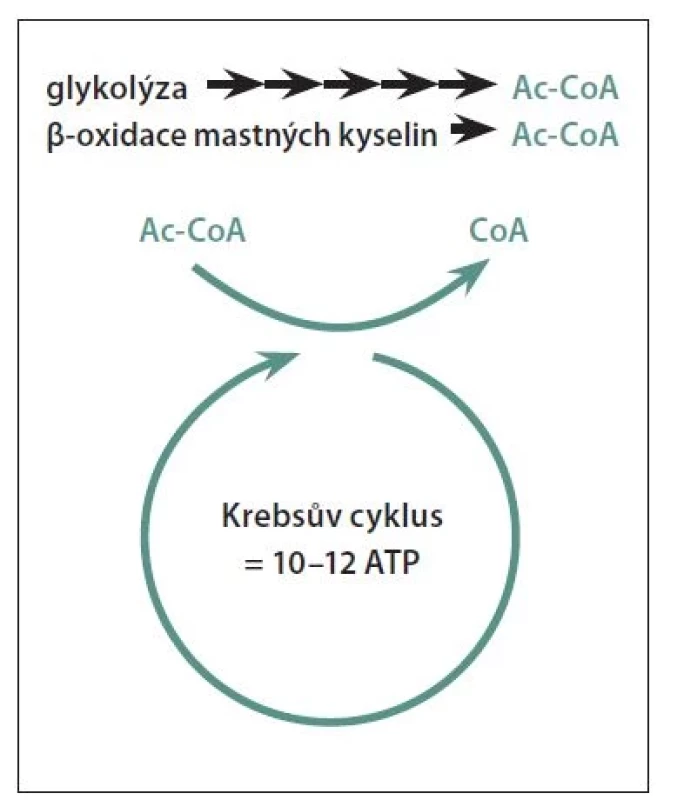
Ac-CoA – acetyl-coenzyme A; ATP – adenosine triphosphate
Low-fat diets
Among the best known specific diets for MS is the one according to Canadian neurologist Roy L. Swank. Swank published in 1950 a direct correlation between the incidence of MS in Norwegian regions and the amount of saturated animal fatty acid intake. This led to the theory of a possible positive effect of a low-fat diet on the course of MS. Supporting his theory was the fact that before the industrial revolution, MS was a relatively rare disease. During the Industrial Revolution between 1750 and 1860, fat consumption in society increased from 60 g to 100 g/day. In 1868, it was named by the French pathologist Charcot [34,35]. It is questionable whether the increased incidence of MS was not simply a result of the recognition and naming of the disease.
In 1950, Swank began testing his hypothesis on 144 MS patients whom he had followed for 34 years. Among the most cited claims from the published results is that among the "good dieters" on the strictest level of a low-fat diet (i.e., below 20 g of fat per day), the fewest patients died after 34 years, 31 vs. 81% for those with fat intakes above 30 g/day. However, it is also noted that the group with the highest mortality rate had almost twice the disease duration at the time of the start of follow-up. It is not stated what the average age of patients in the groups studied was at baseline. On the other hand, the study indicates that when patients were stratified according to duration of illness and age, there was a direct correlation between duration of illness, increase in disability and number of deaths, with no evidence of an effect of fat intake per day. Furthermore, generally greater survival was observed after 34 years in the female sex and in younger patients with shorter disease duration. Patients with fat intake above 20 g/day were designated as "poor dieters", but included 7% of patients who did not develop significant worsening of the disease during the entire follow-up period, while some patients showed a decrease in disability. The average fat intake of this clinically well subgroup was as high as 38 g/day. Swank described the form of MS in these patients as benign [36]. Estimates of the percentage of inherently benign forms of MS vary, further complicating the advent of effective therapy, but estimates of 10% of patients have been reported [37].
The results of the Swank diet are the basis for the holistic treatment protocol "Overcoming MS" by Australian doctor Jelinek. The diet recommends the intake of saturated fat up to 20 g/day, dairy products, meat, palm and coconut fat are prohibited [38]. The effect of this holistic protocol has been shown to improve the quality of life of patients and their families, but the clinical decline in disease activity was not significant [39].
The third of the specific low-fat diets is the McDougall diet, according to an American physician who based his dietary recommendations on observations of the historical health trends of the Hawaiian population. The first immigrants from Asia subsisted primarily on rice and vegetables. It was not until their descendants switched to a hypercaloric, so-called Western diet that the classic diseases of civilization began to manifest themselves [40]. McDougall recommends a vegan high carbohydrate diet with fat intake up to 10% of caloric intake per day. The effect of the diet itself has been noted by McDougall in studies in reducing the risk of cardiovascular and metabolic diseases [41]. In MS patients, reductions in fatigue rates, cholesterol, LDL cholesterol, insulin and body mass index (BMI) levels were found after 12 months of dieting. The results of brain MRI, number of relapses or disability rates did not differ from the control group [42].
Low carbohydrate diets
Common to the diets described below is a low carbohydrate intake offset by a high fat intake to maintain nutrition. We start talking about a low-carbohydrate diet when carbohydrate intake is below 100 g/day. When carbohydrate intake falls below approximately 20-50 g/day, the body begins to use fat metabolites, ketone bodies, for energy production [43] (Figure 1). Assuming that not only the body's fat stores are used (starvation, weight loss), but energy is supplied by regular high fat intake, we speak of a ketogenic diet. The ideal type of low-carbohydrate diet is the Paleo diet, which is based on the assumption that our bodies have not yet adapted to the modern diet resulting from the agricultural and industrial revolution and that we should return to the dietary composition of the hunter-gatherer days, i.e. eat vegetables, fruit, meat optimally from non-domesticated game and avoid dairy products and cereals with gluten. [6].
The goal of the ketogenic diet is to achieve nutritional ketosis, a state where cellular energy production in the form of ATP is provided by blood levels of ketone bodies, not glucose. In neurology, the ketogenic diet first began to be used in the treatment of pharmacoresistant epilepsy in children [44]. Gradually, the physiological actions of ketone bodies in the CNS are being revealed, where they act as potent antioxidants to prevent inflammation, increase ATP production, promote mitochondrial biogenesis, and stabilize the neuronal membrane through potassium channels, thus exerting neuroprotective effects [10]. After 6 months on a ketogenic diet, MS patients experienced an improvement in the gut microbiome [45], a decrease in fatigue, depression, disability, serum pro-inflammatory leptin levels and an improvement in quality of life with an increase in gait [46].
A functional holistic approach to the treatment of MS, including a specific low-carbohydrate diet, is contained in the "Wahls Protocol" by the American physician Terry Wahls. Wahls was diagnosed with MS in 2000 and was unresponsive to therapy. Predominantly using a diet based on the Paleolithic diet, she was able to return to walking and cycling independently after getting into a wheelchair. The essence of the diet is an emphasis on maximizing the intake of micronutrients for the CNS through regular intake of vegetables and fruits in three categories (leafy, colorful, and sulfur-rich), as well as elimination of carbohydrates incl. starchy vegetables, and regular intake of coconut oil, optimally combined with intermittent fasting [47]. Wahls has also subjected his diet to numerous studies in MS patients, in whom it has been shown to alleviate fatigue, improve quality of life, cognition, lipidogram or reduce BMI [48-51]. When the diet is combined with recommended neurostimulation and rehabilitation, improved mobility has been observed in patients with progressive MS [52].
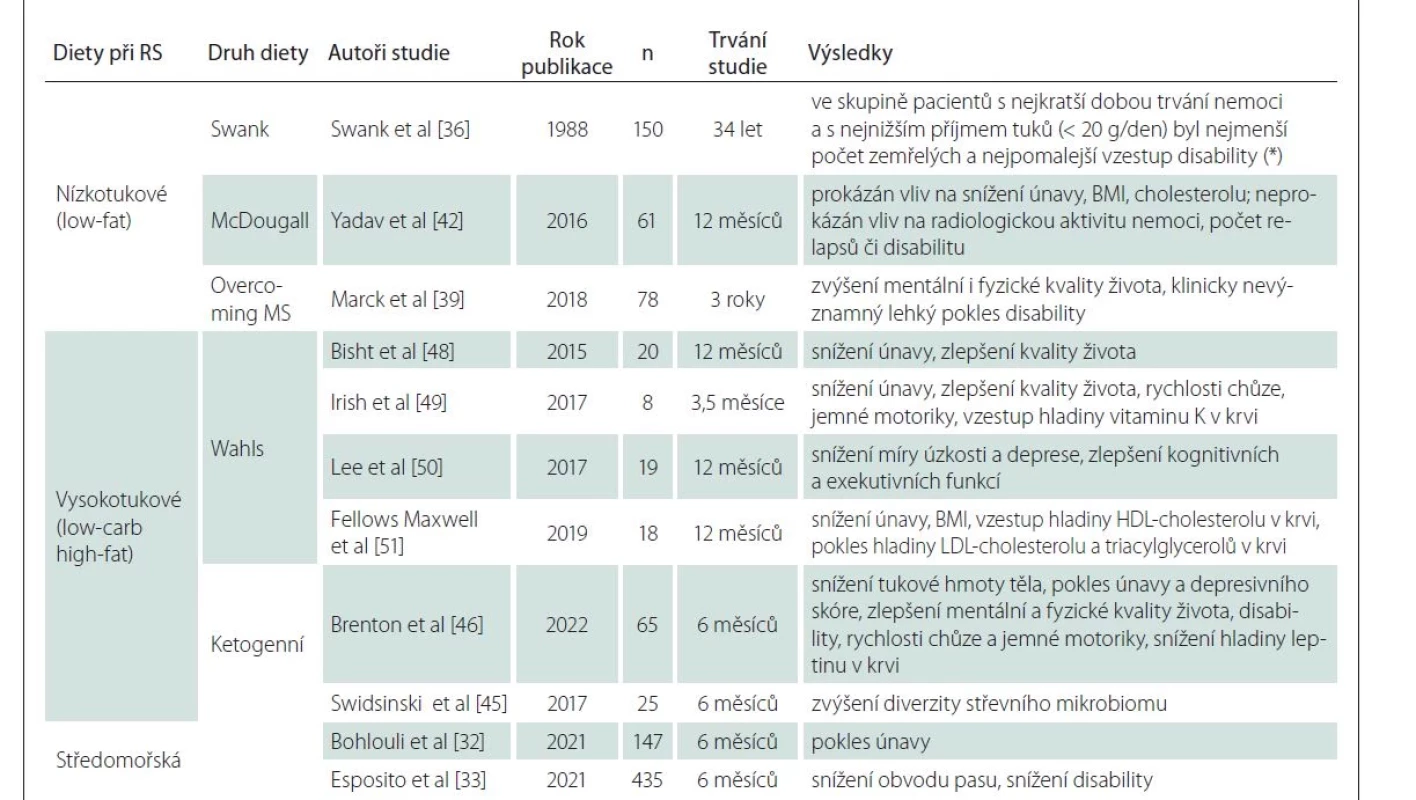
A brief summary of specific diets for MS can be found in Table 1.
Conclusion
To date, there is no single recommended dietary approach for MS patients, nor can it be determined from the above review. In 2021, the results of the long-awaited WAVES study were published, which randomly investigated the effect of two of the most popular, yet completely opposite dietary approaches according to Swank and Wahls in MS patients. Both diets were associated with a decrease in fatigue and improved quality of life. The improvement in walking speed was not significant with either diet, but the Wahls arm showed a significant improvement [53]. Both diets were associated with a significant reduction in the intake of important minerals and vitamins that needed to be supplemented [54]. Thus, although the low-fat diet is based more on theory based on observations of dietary change throughout human history and the high-fat diet on biological understanding of the effects of ketone bodies, the effect on the course of MS was similar.
Rational recommendations for a healthy diet should be followed and patients should pay attention to the quality of their diet. Higher overall diet quality is associated with lower rates of disability, depression, fatigue and cognitive deficits [2]. Emphasis should also be placed on reducing obesity, which is a risk factor for MS incidence, especially in adolescence. The role of pro-inflammatory adipocytokines on disease progression at disease onset has been debated [55]. Every dietary intervention has an impact, positive or negative, on the quality and diversity of the gut microbiome, which then substantially influences the health of the whole body through its metabolites. A better composition of the gut microbiome generally benefits from a reduction in simple sugars and an increase in fibre and healthy fat intake [56]. High salt intake [57], a higher ratio of omega-6 than omega-3 fatty acid intake [58], and a Western diet high in both sugars and fats simultaneously [59] are pro-inflammatory in the body.
Further research is needed to elucidate the mechanisms of dietary interventions to improve the course of MS. So far, almost all diets with beneficial effects are associated with increased intake of a diverse range of vegetables and fruits with different levels of beneficial substances. If patients opt for a specific dietary intervention for MS, additional micronutrient supplementation should be considered and made sustainable in the long term, as better results have been achieved with longer diet duration [53].
Grant support
Supported by Charles University from GAUK 228120 and the Czech Health Research Agency AZV NU20-04-00077.
Conflict of interest
The authors declare that they have no conflict of interest in relation to the subject of the paper.
Tables
Sources
1. Hadgkiss EJ, Jelinek GA, Weiland TJ et al. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci 2015; 18 (3): 125–136. doi: 10.1179/1476830514Y.0000000117.
2. Fitzgerald KC, Tyry T, Salter A et al. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018; 90 (1): e1–e11. doi: 10.1212/WNL.0000000000004768.
3. Leong EM, Semple SJ, Angley M et al. Complementary and alternative medicines and dietary interventions in multiple sclerosis: what is being used in South Australia and why? Complement Ther Med 2009; 17 (4): 216–223. doi: 10.1016/j.ctim.2009.03.001.
4. Katz Sand I. The role of diet in multiple sclerosis: mechanistic connections and current evidence. Curr Nutr Rep 2018; 7 (3): 150–160. doi: 10.1007/s13668-018-0236-z.
5. Schuh C, Wimmer I, Hametner S et al. Oxidative tissue injury in multiple sclerosis is only partly reflected in experimental disease models. Acta Neuropathol 2014; 128 (2): 247–266. doi: 10.1007/s00401-014-1263-5.
6. Langley MR, Triplet EM, Scarisbrick IA. Dietary influence on central nervous system myelin production, injury, and regeneration. Biochim Biophys Acta Mol Basis Dis 2020; 1866 (7): 165779. doi: 10.1016/j.bbadis.2020.165779.
7. Riccio P, Rossano R, Liuzzi GM. May diet and dietary supplements improve the wellness of multiple sclerosis patients? A molecular approach. Autoimmune Dis 2011; 2010 : 249842. doi: 10.4061/2010/249842.
8. Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol 2004; 251 (3): 261–268. doi: 10.1007/s00415-004-0348-9.
9. Waslo C, Bourdette D, Gray N et al. Lipoic acid and other antioxidants as therapies for multiple sclerosis. Curr Treat Options Neurol 2019; 21 (6): 26. doi: 10.1007/s11940-019-0566-1.
10. Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int 2015; 2015 : 681289. doi: 10.1155/2015/681289.
11. Carlson NG, Rose JW. Antioxidants in multiple sclerosis: do they have a role in therapy? CNS Drugs 2006; 20 (6): 433–441. doi: 10.2165/00023210-200620060-00001.
12. Jarrett SG, Milder JB, Liang LP et al. The ketogenic diet increases mitochondrial glutathione levels. J Neurochem 2008; 106 (3): 1044–1051. doi: 10.1111/j.1471-4159.2008.05460.x.
13. Tremlett H, Fadrosh DW, Faruqi AA et al. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur J Neurol 2016; 23 (8): 1308–1321. doi: 10.1111/ene.13026.
14. Esposito S, Bonavita S, Sparaco M et al. The role of diet in multiple sclerosis: a review. Nutr Neurosci 2018; 21 (6): 377–390. doi: 10.1080/1028415X.2017.1303016.
15. Turnbaugh PJ, Ridaura VK, Faith JJ et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 2009; 1 (6): 6ra14. doi: 10.1126/scitranslmed.3000322.
16. Schepici G, Silvestro S, Bramanti P et al. The gut microbiota in multiple sclerosis: an overview of clinical trials. Cell Transplant 2019; 28 (12): 1507–1527. doi: 10.1177/0963689719873890.
17. Furusawa Y, Obata Y, Fukuda S et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013; 504 (7480): 446–450. doi: 10.1038/nature12721.
18. Cignarella F, Cantoni C, Ghezzi L et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab 2018; 27 (6): 1222–1235.e6. doi: 10.1016/j.cmet.2018.05.006.
19. Hedman AM, van Haren NEM, Schnack HG et al. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp 2012; 33 (8): 1987–2002. doi: 10.1002/hbm. 21334.
20. Zivadinov R, Jakimovski D, Gandhi S et al. Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine. Expert Rev Neurother 2016; 16 (7): 777–793. doi: 10.1080/14737175.2016.1181543.
21. Mao P, Reddy PH. Is multiple sclerosis a mitochondrial disease? Biochim Biophys Acta 2010; 1802 (1): 66–79. doi: 10.1016/j.bbadis.2009.07.002.
22. Rothhammer V, Mascanfroni ID, Bunse L et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 2016; 22 (6): 586–597. doi: 10.1038/nm.4106.
23. Xue Z, Li D, Yu W et al. Mechanisms and therapeutic prospects of polyphenols as modulators of the aryl hydrocarbon receptor. Food Funct 2017; 8 (4): 1414–1437. doi: 10.1039/c6fo01810f.
24. Joseph JA, Shukitt-Hale B, Denisova NA et al. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci 1999; 19 (18): 8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999.
25. Xin J, Feinstein DL, Hejna MJ et al. Beneficial effects of blueberries in experimental autoimmune encephalomyelitis. J Agric Food Chem 2012; 60 (23): 5743–5748. doi: 10.1021/jf203611t.
26. Rinholm JE, Hamilton NB, Kessaris N et al. Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci 2011; 31 (2): 538–548. doi: 10.1523/JNEUROSCI.3516-10.2011.
27. Castellano CA, Nugent S, Paquet N et al. Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer‘s disease dementia. J Alzheimers Dis 2015; 43 (4): 1343–1353. doi: 10.3233/JAD-141074.
28. Kindred JH, Tuulari JJ, Bucci M et al. Walking speed and brain glucose uptake are uncoupled in patients with multiple sclerosis. Front Hum Neurosci 2015; 9 : 84. doi: 10.3389/fnhum.2015.00084.
29. Kuhlmann T, Miron V, Cui Q et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 2008; 131 (Pt 7): 1749–1758. doi: 10.1093/brain/awn 096.
30. Bai M, Wang Y, Han R et al. Intermittent caloric restriction with a modified fasting-mimicking diet ameliorates autoimmunity and promotes recovery in a mouse model of multiple sclerosis. J Nutr Biochem 2021; 87 : 108493. doi: 10.1016/j.jnutbio.2020.108 493.
31. Román GC, Jackson RE, Gadhia R et al. Mediterranean diet: the role of long-chain omega-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev Neurol (Paris) 2019; 175 (10): 724–741. doi: 10.1016/j.neurol.2019.08.005.
32. Bohlouli J, Namjoo I, Borzoo-Isfahani M et al. Modified Mediterranean diet v. traditional Iranian diet: efficacy of dietary interventions on dietary inflammatory index score, fatigue severity and disability in multiple sclerosis patients. Br J Nutr 2022; 128 (7): 1274–1284. doi: 10.1017/S000711452100307X.
33. Esposito S, Sparaco M, Maniscalco GT et al. Lifestyle and Mediterranean diet adherence in a cohort of Southern Italian patients with multiple sclerosis. Mult Scler Relat Disord 2021; 47 : 102636. doi: 10.1016/j.msard.2020.102636.
34. Swank RL. Multiple sclerosis; a correlation of its incidence with dietary fat. Am J Med Sci 1950; 220 (4): 421–430.
35. Swank RL, Goodwin J. Review of MS patient survival on a Swank low saturated fat diet. Nutrition 2003; 19 (2): 161–162. doi: 10.1016/s0899-9007 (02) 00851-1.
36. Swank RL, Grimsgaard A. Multiple sclerosis: the lipid relationship. Am J Clin Nutr 1988; 48 (6): 1387–1393. doi: 10.1093/ajcn/48.6.1387.
37. Noseworthy JH, Lucchinetti C, Rodriguez M et al. Multiple sclerosis. N Engl J Med 2000; 343 (13): 938–952. doi: 10.1056/NEJM200009283431307.
38. Overcoming MS. Our history & context. [online]. Dostupné z: https: //overcomingms.org/about-us/our-history-context.
39. Marck CH, De Livera AM, Brown CR et al. Health outcomes and adherence to a healthy lifestyle after a multimodal intervention in people with multiple sclerosis: three year follow-up. PLoS One 2018; 13 (5): e0197759. doi: 10.1371/journal.pone.0197759.
40. Dr. McDougall. Our story. [online]. Dostupné z: https: //www.drmcdougall.com/our-story/.
41. McDougall J, Thomas LE, McDoufall C et al. Effects of 7 days on an ad libitum low-fat vegan diet: the McDougall Program cohort. Nutr J 2014; 13 : 99. doi: 10.1186/1475-2891-13-99.
42. Yadav V, Marracci G, Kim E et al. Low-fat, plant-based diet in multiple sclerosis: a randomized controlled trial. Mult Scler Relat Disord 2016; 9 : 80–90. doi: 10.1016/j.msard.2016.07.001.
43. Adam-Perrot A, Clifton P, Brouns F. Low-carbohydrate diets: nutritional and physiological aspects. Obes Rev 2006; 7 (1): 49–58. doi: 10.1111/j.1467-789X.2006.002 22.x.
44. Sourbron J, Klinkenberg S, van Kuijk SMJ et al. Ketogenic diet for the treatment of pediatric epilepsy: review and meta-analysis. Childs Nerv Syst 2020; 36 (6): 1099–1109. doi: 10.1007/s00381-020-04578-7.
45. Swidsinski A, Dörffel Y, Loening-Baucke V et al. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. Front Microbiol 2017; 8 : 1141. doi: 10.3389/fmicb.2017.01141.
46. Brenton JN, Lehner-Gulotta D, Woolbright E et al. Phase II study of ketogenic diets in relapsing multiple sclerosis: safety, tolerability and potential clinical benefits. J Neurol Neurosurg Psychiatry 2022; 93 (6): 637–644. doi: 10.1136/jnnp-2022-329074.
47. Wahls T. The Wahls protocol: a radical new way to treat all chronic autoimmune conditions using paleo principles. Baltimore: Penguin Publishing Group 2014.
48. Bisht B, Darling WG, Torage Shivapour E et al. Multimodal intervention improves fatigue and quality of life in subjects with progressive multiple sclerosis: a pilot study. Degener Neurol Neuromuscul Dis 2015; 5 : 19–35. doi: 10.2147/DNND.S76523.
49. Irish AK, Erickson CM, Wahls TL et al. Randomized control trial evaluation of a modified Paleolithic dietary intervention in the treatment of relapsing-remitting multiple sclerosis: a pilot study. Degener Neurol Neuromuscul Dis 2017; 7 : 1–18. doi: 10.2147/DNND.S116949.
50. Lee JE, Bisht B, Hall MJ et al. A multimodal, nonpharmacologic intervention improves mood and cognitive function in people with multiple sclerosis. J Am Coll Nutr 2017; 36 (3): 150–168. doi: 10.1080/07315724.2016.1255160.
51. Fellows Maxwell K, Wahls T, Browne RW et al. Lipid profile is associated with decreased fatigue in individuals with progressive multiple sclerosis following a diet - -based intervention: results from a pilot study. PLoS One 2019; 14 (6): e0218075. doi: 10.1371/journal.pone.0218075.
52. Wahls TL, Reese D, Kaplan D et al. Rehabilitation with neuromuscular electrical stimulation leads to functional gains in ambulation in patients with secondary progressive and primary progressive multiple sclerosis: a case series report. J Altern Complement Med 2010; 16 (12): 1343–1349. doi: 10.1089/acm.2010.0080.
53. Wahls TL, Titcomb TJ, Bisht B et al. Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: the WAVES randomized parallel-arm clinical trial. Mult Scler J Exp Transl Clin 2021; 7 (3): 20552173211035399. doi: 10.1177/20552173211035399.
54. Titcomb TJ, Brooks L, Smith KL et al. Change in micronutrient intake among people with relapsing-remitting multiple sclerosis adapting the Swank and Wahls diets: an analysis of weighed food records. Nutrients 2021; 13 (10): 3507. doi: 10.3390/nu13103507.
55. Schreiner TG, Genes TM. Obesity and multiple sclerosis – a multifaceted association. J Clin Med 2021; 10 (12): 2689. doi: 10.3390/jcm10122689.
56. Moszak M, Szulinska M, Bogdanski P. You are what you eat – the relationship between diet, microbiota, and metabolic disorders – a review. Nutrients 2020; 12 (4): 1096. doi: 10.3390/nu12041096.
57. Hucke S, Wiendl H, Klotz L. Implications of dietary salt intake for multiple sclerosis pathogenesis. Mult Scler 2016; 22 (2): 133–139. doi: 10.1177/1352458515609431.
58. Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids 2018; 132 : 41–48. doi: 10.1016/j.plefa.2018.03.004.
59. Matveeva O, Bogie JFJ, Hendriks JJA et al. Western lifestyle and immunopathology of multiple sclerosis. Ann N Y Acad Sci 2018; 1417 (1): 71–86. doi: 10.1111/ nyas.13583.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2023 Issue 1
-
All articles in this issue
- Editorial
- Poděkování recenzentům
- Progressive multiple sclerosis in the light of the latest findings
- Recommendations for structural brain MRI in the diagnosis of epilepsy
- Dietary approaches specific to patients with multiple sclerosis
- Stroke specific measurement tools used to assess health related quality of life in young adults after ischemic stroke
- The role of dynamic MRI of the cervical spine and dynamic evoked potentials in the diagnosis of degenerative cervical myelopathy
- Validation of the Electronic Memory Test ALBAV
- Psychopathological context of alexithymia in patients with back pain
- Narcolepsy severity scale and its psychometric properties in patients with narcolepsy type 1 in the Czech Republic
- Decompressive craniectomy with watertight duroplasty and without watertight duroplasty - advantages and disadvantages
- The first Czech patient with aminoacylase I deficiency
- Komentář k článku autorů Kövári et al Ovlivnění spasticity pomocí elektrické stimulace podle Jantsche – pilotní studie
- Komentář k článku autorů Ehler et al Onemocnění bederní páteře – nová neurologická nemoc z povolání
- Doc. MUDr Zbyněk Kalita, CSc. dovršil 80 let zdařilého života
- Free serum triiodothyronine associations with the Mini-Mental State Examination score after experienced acute ischemic stroke
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Progressive multiple sclerosis in the light of the latest findings
- Recommendations for structural brain MRI in the diagnosis of epilepsy
- Dietary approaches specific to patients with multiple sclerosis
- The role of dynamic MRI of the cervical spine and dynamic evoked potentials in the diagnosis of degenerative cervical myelopathy