Evaluation of systolic and diastolic cardiac functions and heart rate variability in patients with juvenile myoclonic epilepsy
Vyhodnocení systolických a diastolických srdečních funkcí a variability srdeční frekvence u pacientů s juvenilní myoklonickou epilepsií
Cíl:
Prozkoumat, zda u pacientů s juvenilní myoklonickou epilepsií (JME) existují rozdíly ve variabilitě srdeční frekvence (heart rate variability; HRV) spojené s rizikem arytmií a v systolických a diastolických funkcích.
Soubor a metodika:
Tato jednocentrická prospektivní studie zahrnovala 50 pacientů s JME, kteří byli sledováni na ambulanci pro epilepsii Neurologického oddělení nemocnice Antalya Training and Research Hospital (34 žen, průměrný věk 26 ± 7,58 let), a 45 zdravých kontrolních osob (30 žen, průměrný věk 26,71 ± 5,14 let). Dva pacienti byli ze studie vyloučeni z důvodu arteriální hypertenze, jeden pacient byl vyloučen kvůli diabetu mellitu a jeden pacient byl vyloučen kvůli revmatické mitrální stenóze. Nakonec bylo do studie zařazeno 46 pacientů. U všech pacientů a kontrolních osob byly konvenční echokardiografií a tkáňovým dopplerovským zobrazením vyšetřeny systolické a diastolické funkce (např. ejekční frakce levé komory, rozměry a objemy levé komory, decelerační čas, plicní dopředné toky) a provedl se 24h Holterův monitoring za účelem prozkoumání parametrů časové domény (např. směrodatná odchylka intervalů „normal-to-normal“, druhá odmocnina průměru druhých mocnin rozdílů mezi po sobě jdoucími intervaly „normal-to-normal“) a parametrů frekvenční domény HRV.
Výsledky:
Mezi oběma skupinami nebyly zjištěny žádné významné rozdíly v Holterovských parametrech, pokud jde o HRV. Echokardiografické vyšetření neodhalilo žádné významné rozdíly vyjma vzájemného poměru plicního venózního systolického a diastolického (PVS/PVD) dopředného toku (p = 0,008).
Závěr:
V této studii jsme u pacientů s JME kromě zvýšeného poměru PVS/PVD nenalezli nic jiného, co by se týkalo srdečního postižení, a rovněž jsme u nich nepozorovali autonomní dysfunkci. Tato skutečnost může být důsledkem dobré kontroly záchvatů.
Klíčová slova:
variabilita srdeční frekvence – juvenilní myoklonická epilepsie – srdeční funkce – echokardiografie
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors:
F. Genç 1; A. Genç 2; E. Küçükseymen 1; A. Erdal 1; Y. B. Gömceli 1; Ş. Arslan 2; G. Kutlu 3
Authors‘ workplace:
Department of Neurology, Antalya Training and Research Hospital, Antalya, Turkey
1; Department of Cardiology, Antalya Training and Research Hospital, Antalya, Turkey
2; Department of Neurology and Clinical Neurophysiology, Faculty of Medicine, Muğla Sıtkı Koçman University, Muğla, Turkey
3
Published in:
Cesk Slov Neurol N 2018; 81(6): 700-706
Category:
Original Paper
doi:
https://doi.org/10.14735/amcsnn2018700
Overview
Aim:
To explore if patients with juvenile myoclonic epilepsy (JME) have differences in heart rate variability (HRV) associated with risk of arrhythmias and in systolic and diastolic functions.
Patients and methods:
This single-centre prospective study included 50 patients with JME followed up at the epilepsy outpatient clinic within the Antalya Training and Research Hospital’s Neurology Department (34 women, mean age 26 ± 7.58 years) and 45 healthy controls (30 women, mean age 26.71 ± 5.14 years). Two patients were excluded since they had arterial hypertension, one patient was excluded due to diabetes mellitus and one patient was excluded due to rheumatic mitral stenosis. Finally, 46 patients were included in the study. All patients and controls were evaluated by conventional echocardiography and tissue Doppler imaging for systolic and diastolic functions (e. g. left ventricular ejection fraction, left ventricle diameters and volumes, deceleration time, pulmonary forward flows) and performing 24-h Holter monitoring to explore time domain (e. g. standard deviation of the normal-to-normal interval, the square root of the mean squared differences of successive normal-to-normal intervals) and frequency domain parameters of HRV.
Results:
There were no significant differences between the Holter parameters of the two groups with respect to HRV. Echocardiographic investigation did not reveal any significant differences except for the ratio of pulmonary venous systolic and diastolic (PVS/ PVD) forward flows to one another (p = 0.008).
Conclusion:
In this study, we did not find anything else about cardiac involvement other than increased ratio of PVS/ PVD and we did not find autonomic dysfunction in patients with JME. This may be due to good seizure control.
Key words:
heart rate variability – juvenile myoclonic epilepsy – cardiac functions – echocardiography
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Chinese summary - 摘要
青少年肌阵挛性癫痫患者收缩期和舒张期心功能及心率变异性的评估
目标:
探讨青少年肌阵挛性癫痫(JME)患者心率变异性(HRV)与心律失常风险以及收缩和舒张功能的差异。
患者和方法:
这项单中心前瞻性研究纳入了50名JME患者,随后在安塔利亚培训和研究医院神经内科的癫痫门诊纳入(34名女性,平均年龄26±7.58岁)和45名健康对照(30名女性,平均年龄26.71± 5.14岁)。两名患者因动脉高血压被排除在外,一名患者因糖尿病而被排除,另一名患者由于风湿性二尖瓣狭窄而被排除。最后,46名患者被纳入研究。通过常规超声心动图和组织多普勒成像评估所有患者和对照的收缩和舒张功能(例如左心室射血分数,左心室直径和体积,减速时间,肺前向流动)并进行24小时动态心电图监测以探索时域(例如,正常到正常间隔的标准偏差,连续正常到正常间隔的均方差的平方根)和HRV的频域参数。
结果:
两组的Holter参数与HRV无显著差异。除了肺静脉收缩压和舒张压(PVS / PVD)相互之间的流量比(p = 0.008),超声心动图检查未发现任何显著差异。
结论:
在这项研究中,除了增加PVS / PVD的比例之外,我们没有发现任何关于心脏牵涉的信息,我们在JME患者中没有发现自主神经功能障碍。 这可能是由于良好的癫痫发作控制。
关键词:
心率变异性 - 青少年肌阵挛性癫痫 - 心脏功能 - 超声心动图
Introduction
Epilepsy often affects the autonomic functions during ictal, inter-ictal and postictalperiods. Epileptic discharges are inculpated for impairing or altering the central autonomic pathway and normal autonomic cardiac functions in patients having seizures. Sudden unexpected death in epilepsy (SUDEP) is the leading cause of death in patients with epilepsy and accounts for about 17% of these deaths [1]. However, the mechanisms involved in SUDEP are not well understood; fatal cardiac arrhythmias caused by the activation of the autonomic nervous system (ANS) are also inculpated. Measurement of heart rate variability (HRV) is one of the ways to measure the effects of ANS on the heart. The intervals between the heart beats in the sinus rhythm are different in healthy individuals, which is a normal physiological process [2]. HRV refers to the mathematical formulation of the fluctuations in heart rate around the average heart rate within a certain time [2]. Physical and metabolic factors lead to variations in the heart rate associated with autonomic tonus. HRV seems to be very promising for the investigation of cardiovascular response to the variations in the autonomic tonus [2,3].Increased activation of the sympathetic system and suppression of the parasympathetic system leads to a decline in the HRV [2]. HRV is evaluated in two ways: time and frequency analysis. The intervals between the normal pulses in the 24-h electrocardiography (ECG) records are analyzed during the time domain measurement, while the heart rate signals are grouped according to frequencies and intensities and information is obtained about the amount of heart rate variation on the basis of the periodical cardiac oscillations at different frequencies during the frequency domain spectral analysis [2,4]. In our study, we investigated autonomic dysfunction through time domain and frequency domain HRV analysis in patients diagnosed with juvenile myoclonic epilepsy (JME) and healthy controls. Recent studies found that epilepsy patients had cardiac systolic and diastolic dysfunctions, while the effect of ANS on the heart are inculpated for such abnormalities [5].
Increased sympathetic cardiac stimulation may lead to cardiac arrhythmias but also repetitive sympathetic stimulation may cause structural damage in the heart, which means that there is a high predisposition to arrhythmia and ischaemia. Myocardial fibrosis may also lead to left ventricular systolic and diastolic dysfunction. We evaluated the presence of such dysfunction through conventional echocardiographic investigation and tissue Doppler imaging (TDI).
Patients and methods
This single-centre prospective study enrolled 50 patients with JME followed up at the epilepsy outpatient clinic within the Antalya Training and Research Hospital’s Neurology Department (34 women, mean age 26 ± 7.58 years) and 45 healthy controls (30 women, mean age 26.71 ± 5.14 years). Patients were diagnosed by three neurologists who were specialized in epilepsy. The neurological examination of the patients and controls was performed by that team of neurologists and their cardiologic examination was performed by a cardiologist.
Exclusion criteria included pregnancy, known coronary artery disease, hepatic andrenal dysfunction, arterial hypertension, valvular heart disease, left ventricular systolic dysfunction (ejection fraction <50%), restrictive, hypertrophic or dilated cardiomyopathy, congenital cardiac disease, pulmonary pathologies, diabetes mellitus, malignancies, severe alcohol consumption, hyperlipidaemia, previous cardiac surgery, severe mitral annular calcification, atrial fibrillation or other severe arrhythmias, use of pacemaker or implantable cardioverter defibrillator, poor echocardiographic image quality. Two patients were excluded since they had arterial hypertension, one patient was excluded due to diabetes mellitus and one patient was excluded due to rheumatic mitral stenosis and thus 46 patients were included in the study and 40 of them were seizure-free.
The epilepsy diagnosis was established on the basis of the Guidelines of the International League Against Epilepsy, and the patients were diagnosed according to the medical history of the patients and their relatives, physical and neurological examination, as well as the EEG and neuroradiological findings [6].
Blood pressure of both groups was measured in sitting position after 5-min rest. Height, weight and waist circumference of both groups were measured and their body mass index and body surface area were calculated. Potential SUDEP risk in patients was estimated using an inventory of seven validated SUDEP risk factors (SUDEP-7), which was assembled from a large prospective cohort study of SUDEP reported by Walczak et al. [7]. Based on this study, Di Giorgo et al. validated a SUDEP-7 inventory [8]. This inventory consists of seven items in which scores were based on the log of the odds ratio of the main risk factors [7,8]. The score on the SUDEP-7 ranged from 1 to 7, out of a maximum possible score of 10.
Electrocardiographic study
Standard 12-lead ECG was recorded in all participants to study the following variables: heart rate, PR interval (the time between the beginning of P-wave and the beginning of QRS complex), QRS complex duration, QT interval (the time between the beginning of QRS complex and the end of T-wave), QTc interval (the QT interval after correction for heart rate, which is the QT interval divided by the square root of RR interval [Bazett’s formula]).
Echocardiography study
The echocardiographic investigation of all patients and the control group was performed using Philips IE33 xMatric (Philips, Andover, MA, USA) with 5-1MHz matrix transducer using transthoracic approach. The images of the patients were obtained in left lateral decubitus position, while measurements were performed in 2D, M Mode, continuous-wave Doppler, pulse-wave Doppler and TDI echocardiography using parasternal long and short axis, apical four-chamber and five-chamber views. Left ventricular and right atrium diameters as well as interventricular septum and posterior wall thickness were measured using M mode technique in line with the recommendations of the American Society of Echocardiography [9]. The early (E) and late (A) peak velocities of diastolic filling, deceleration time (DT) and isovolumetric relaxation time (IVRT) were obtained from mitral inflow and aortic outflow Doppler records [10]. The filter setting was reduced and Nyquist limit was adjusted for TDI measurement (range 15– 20 cm/ s). The gain of the device was reduced in order to decrease the noise in the background and obtain a clear tissue signal. For TDI measurements to be performed from the apical window, 5- mm sample volume was placed lateral to the mitral valvular annulus on 4-chamber view [10]. Measurements were performed simultaneously with ECG at a rate of 50– 100 mm/ s. Peak systolic velocity (s’), early (e’) and late (a’) diastolic velocities and ratio of e’/ a’ were measured. For recording pulmonary venous flow velocities, right superior pulmonary venous flow in the apical four-chamber view was used and sample volume was placed 1– 2 cm into the orifice. Here, pulmonary venous systolic forward flow (PVS) and pulmonary venous diastolic forward flow (PVD) were recorded. The ratio of E/ e’ was obtained by dividing transmitral E peak to e’. The diagnosis and staging of left ventricular diastolic dysfunction (LVDD) were done according to the recommendations of the European Society of Echocardiography [11]. LVDD was diagnosed when lateral mitral annular e’ velocity was lower than 10 cm/ s or left atrium volume index was greater than 34 mL/ m². LVDD has 3 stages (I, II and III). When LVDD was diagnosed, Doppler parameters such as E/ A ratio, DT, IVRT, E/ e’ ratio and PVS/ PVD ratio were used for staging [11]. Ejection fraction was quantified according to Teicholz formula [12]. All echocardiographic investigations were recorded digitally so that they could be examined off-line later. All measurements were performed by calculating the average of three cardiac cycles in order to decrease the respiratory variation.
Heart rate variability
In order to evaluate HRV of the patients and controls, 24-h ECG records were performed using 3-channel digital Holter recording device DMS300-3A (DM Software Inc., Stateline, NV, USA). Holter records were analyzed using the software Cardioscan 12,0 (DM Software Inc., Stateline, NV, US) and the records were evaluated manually in order to rule out the artifacts (ectopic beats, arrhythmic events, missing data and noise effects).
During HRV evaluation, standard deviation (SD) of the normal-to-normal (NN) interval (SDNN), the square root of the mean squared differences of successive NN intervals (RMSSD), the division of the number of interval differences of successive NN intervals of more than 50 ms by the total number of NN intervals (pNN50) were analyzed for the time domain parameters. SDNN represents a global measure of HRV. RMSSD is considered a powerful measure of high frequency (HF) variations in short-term recording, as it provides a useful evaluation of HF and vagal tone [2]. The fast Fourier transformation (FFT) has been used to calculate the power spectrum density. Total power (S POWER) in the range 0.0033–0.4 Hz, power in very low frequency (VLF) in the range 0.0033–0.04 Hz, power in low frequency (LF) in the range 0.04–0.15 Hz and power in high frequency (HF) in the range 0.15–0.4 Hz. were analyzed for frequency domain parameters. VLF was evaluated although this component does not have a well-defined physiological explanation [2]. The HF was regarded as a measure of solely parasympathetic activity. The LF was considered to be a measure of mainly sympathetic activity that was modulated by the influence of the parasympathetic system. LF/ HF ratio expresses the balance between sympathetic and parasympathetic nervous system activity. All analyses were performed according to the standards set by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [2].
This study was conducted upon the local approval no 46/ 12 and dated September 11, 2014 of the ethics board of Antalya Training and Research Hospital, and written consent forms were obtained from everyone included in the study. This study was conducted in compliance with the Declaration of Helsinki.
Statistical analysis
All of the statistical analyses were performed using the SPSS software package, version 21.0 (SPSS Inc., Chicago, IL, USA). The continuous variables were expressed as mean ± SD and the categorical variables as numbers and percentages. The Kolmogorov- Smirnov test was used to determine whether the data were normally distributed. The Mann- Whitney U test was used for variables that were not normally distributed. Statistical significance was defined as p < 0.05.
Results
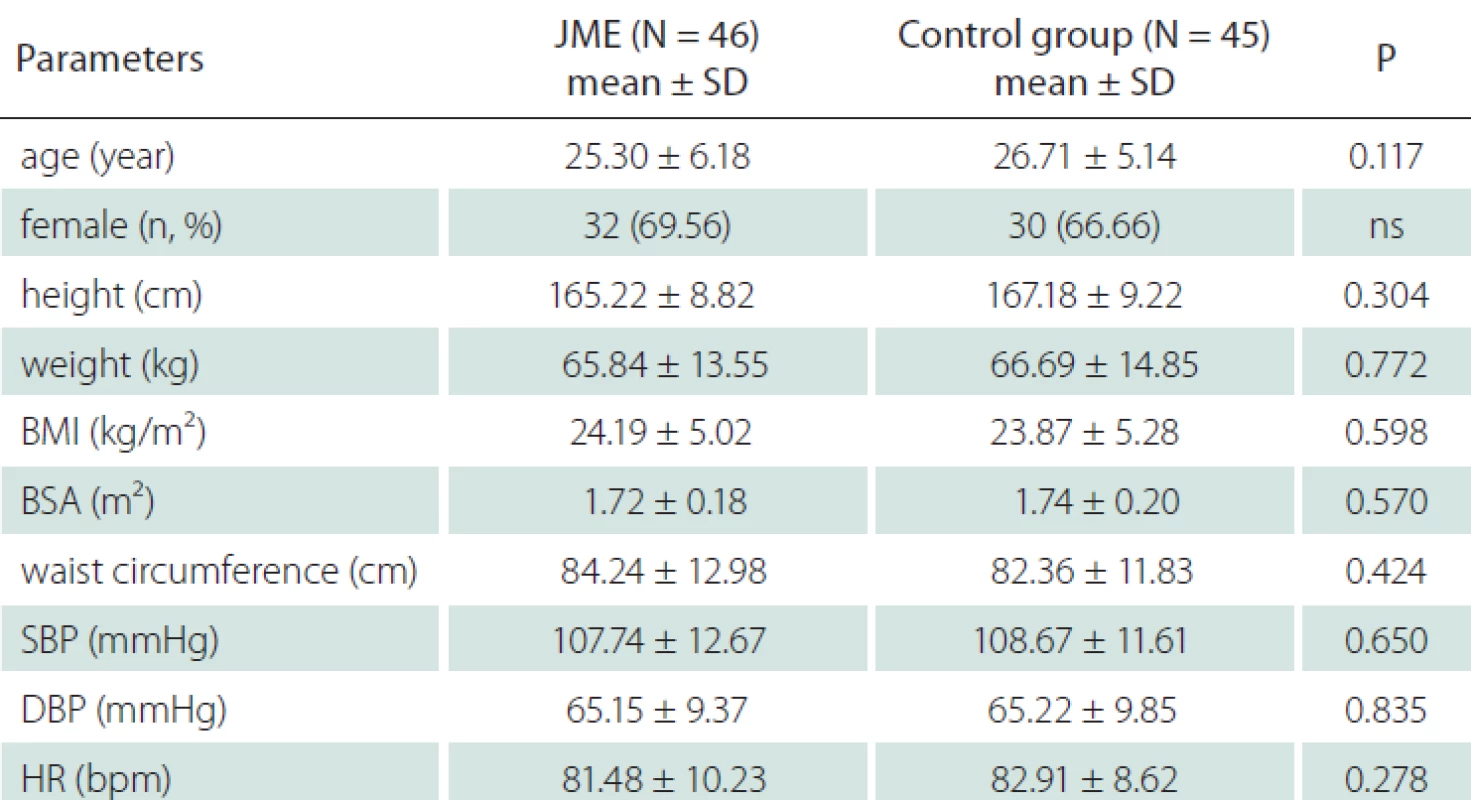
All participants were Caucasians. Thirty-two patients (69.56%) out of 46 included in the study were female and the mean age was 25.30 ± 6.18 years. In the control group, 30 individuals (66.66%) were female and the mean age was 26.71 ± 5.14 years while the control group included 45 healthy individuals. There was no difference between the two groups with respect to height, weight, body mass index, body surface area, waist circumference, systolic and diastolic blood pressure and resting heart rate (Tab. 1).
In JME group, 24 patients did not have any issues in their history; however, 6 patients had a history of febrile convulsion, one had a history of hypoxic delivery, one had a history of CNS infection, 11 had a head trauma and three had a history of febrile convulsion and head trauma. Thirty-two patients did not have any issues in their family history, whereas 13 patients had a family history of epilepsy and the parents of one patient had consanguineous marriage. The mean epilepsy duration was 10.43 ± 6.67 years while the mean duration of drug use was 8.43 (max. 27, min. 1) years, the average frequency of seizures was 0.25 ± 0.7(max. 4, min. 0) per month. For the epileptic treatment, 20 patients were taking valproic acid, 10 patients were taking lamotrigine, 7 patients were taking levetiracetam, 1 patient was taking topiramate, 6 patients were taking dual combination of these medications while 2 patients were on triple combination, mean SUDEP-7 inventory score of patients was 0.19 (max. 2, min. 0). Tab. 2 shows the components of the SUDEP-7 inventory and the number of subjects with each factor.
![The SUDEP risk inventory (SUDEP-7, version 2.0) with each risk factor, weighting, and scoring convention [35].](https://www.csnn.eu/media/cache/resolve/media_object_image_small/media/image_pdf/40f4af583f466045d8b50cf76a3a74ca.png)
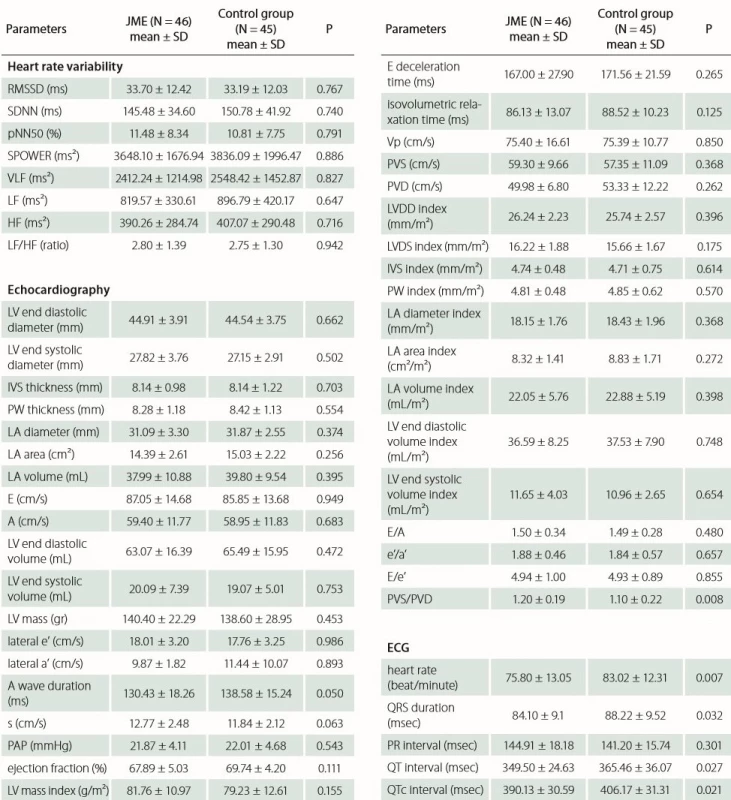
Echocardiography records of both groups and 24-h rhythm Holter were analyzed and registered. There was no significant difference between the JME group and control group with respect to time domain (SDNN, RMSSD, pNN50) and frequency domain (S POWER, VLF, LF, HF, LF/ HF) parameters of HRV. No statistically significant difference was found with respect to left atrium diameter, area and volume index (p = 0.374, p = 0.256 and p = 0.398, resp.); likewise, interventricular septum and posterior wall thickness were similar in both groups (p = 0.703 and p = 0.554, resp.); moreover, there was no statistical difference between the other anatomic echocardiographic values. Furthermore, there was no difference between the Doppler and TDI parameters such as E wave velocity, E/ A ratio, DT, IVRT, e’/ a’ ratio, E/ e’ ratio except PVS/ PVD (p = 0.008). Analysis showed that JME patients had a significantly lower mean of heart rate, QRS duration, QT and QTc interval (p = 0.007, p = 0.032, p = 0.027, p = 0.021 resp.). Tab. 3 presents the HRV, echocardiographic and ECG data of the patient and control groups.
Discussion
Sinus arrhythmia refers to the respiratory cyclic changes in the heart rate and is associated with the cardio-respiratory connection in the brain stem. Physiological HRV is the indication of cardiac health while its absence or reduction may be a sign of a disease or aging process [13]. HRV analysis is a strong tool that gives semi-quantitative information about the relationship between the cardiovascular sympathetic and parasympathetic modulation under several physiological and pathophysiological conditions [14].
Compared to the healthy controls, patients with chronic temporal lobe epilepsy were found to have declined HRV. Although the results suggest that there is a decrease in theparasympathetic tonus and/ or increase in the sympathetic tonus, the data about the re-lationship between epilepsy and the variations in the sympathetic and parasympathetic tonus are still insufficient [15]. We did not find HRV changes in our JME group which may be due to differences in anti-epileptic drugs (AEDs) such as classical sodium blockers that are used in temporal lobe epilepsy but are not used in JME. Increased sympathetic tone of the heart rate may play an important role in the development of ventricular tachycardia that may be associated with the high incidence of SUDEP in generalized tonic clonic seizure (GTCS) patients compared to the control group. Recently, a meta-analysis of HRV in epilepsy reported a lack of significant alterations of LF in epilepsy patients compared to controls; however, this parameter was found to be lower in patients with AEDs when compared to drug-free subjects [16]. As mentioned in the SUDEP-7 inventory that was designed to analyze risk factors identified by Walczak et al., seizure frequency is a well-known risk factor for SUDEP, but recent studies reported that seizure frequency of GTCS is much more important than for patients with any other type of seizure [7,17]. In the SUDEP-7 inventory, results indicated that older age, longer duration of epilepsy, and presence of developmental disability had direct influence on vagus-mediated HRV and thus increased SUDEP risk. The higher the SUDEP-7 inventory score, the higher the risk of SUDEP. Lower RMSSD values were associated with higher risk scores on the new SUDEP risk inventory. This provides new evidence that HRV (specifically RMSSD) is a marker of SUDEP risk [8].
Heart rate variability is observed to vary across the treated and untreated patients. Haliloğlu et al. found in their study that children taking valproic acid, oxcarbazepine or phenobarbital had better HRV results compared to the untreated ones [18]. Contrary to this finding, we did not find any difference with respect to heart rate and HRV parameters, which might be due to the fact that the seizures were controlled well. In a recent trial, comparison of changes in HRV parameters in the peri-apnea / hypopnea period of patients with JME and healthy controls did not show any significant difference except for SDNN [19]. However we did not find any significant difference between JME patients and healthy controls in none of HRV parameters. Further studies using a larger sample size and in patients with newly diagnosed and/ or drug-resistant epilepsy may prove beneficial in understanding the mechanism.
Mechanisms underlying SUDEP may lead to fatal arrhythmia or ischaemia in patients with or without predisposing functional or structural cardiac abnormalities. However, malignant cardiac arrhythmias have rarely been seen during recorded SUDEP cases [20]. Increased sympathetic cardiac stimulation may lead to cardiac arrhythmias but also repetitive stimulation may cause structural damage in the heart, which means that there is a high predisposition to arrhythmia and ischaemia. It was found previously that patients with SUDEP had myocardial fibrosis [21]. Myocardial fibrosis may lead to left ventricular systolic and diastolic dysfunction. Troponin does not rise in the post-ictal period in patients with non-complicated seizures, while ischaemia findings on ECG and elevated cardiac enzymes suggest that epileptic patients may have secondary cardiac damage [22,23]. Alehan et al. showed that brain natriuretic peptide (BMP) and creatine kinase-muscle/brain (CK-MB) were elevated in postictal period in patients with seizures and they obtained the evidence proving that epileptic patients may had mild cardiac dysfunction which was difficult to detect [24]. Elevated sympathetic activity may lead to left ventricular dilatation which may result in Takotsubo and stress-induced cardiomyopathy reported in GTCS. This may affect the cardiac output and lead to insufficient peri-ictal oxygen supply [25,26]. Two studies reporting that recurrent Takotsubo cardiomyopathy developed due to GTCS and convulsive status epilepticus were published [27,28]. The cited authors stated that this occurred due to the sympathetic fluctuations. Bilgi et al. reported that patients with GTCS had not only systolic and diastolic dysfunction but had also impaired end-systolic left ventricular diameter and volume [5]. In a recently published study, increased left atrial diameter was reported in patients with GTCS compared to the control group. The authors argued that this might be caused by the increased left ventricular end-systolic pressure due to the sympathetic activity [29]. Pulmonary vein Doppler method contains load-dependent parameters and thus can be used for the estimation of left ventricular filling pressures [30]. Kuecherer et al. thought that increased left atrial pressure was negatively correlated with PVS and PVS2/ PVD ratio in patients with pseudo normal or restrictive pattern [31]. In our study, we did not find a negative change in PVS/ PVD either, similar to other echocardiographic parameters.
Cardiac ion channels are subdivided as depolarizing and repolarizing ones. Prolonged repolarization can cause triggered activity, while rapid repolarization may lead to reentrant excitation. Eric et al. reported decreased heart rate from 80 to 67 and increased QRS intervals in Lamotrigine users [32]. In our study, we could have detected decreased heart rate in the patient group because 10 of our patients were using LTG but decreased QRS intervals could be due to other AEDs effects. In the line of our study, Ramadan et al. reported shortening of QT and QTc intervals in newly diagnosed GTCS patients without medication [29]. There are, however, conflicting data on whether QTc intervals in epileptic patients differ from controls [33,34]. A potential confounder in these studies could be, at least partly, the use of different AEDs.
Further studies are needed using a larger sample size and in patients with newly diagnosed and/ or drug-resistant JME.
Conclusion
Epilepsy patients may be predisposed to autonomic dysfunctions which may be associated with cardiac arrhythmias due to the effects of recurrent seizures on the cardiac microstructure. There are several studies demonstrating the effect of epilepsy on cardiac and autonomic functions. However, studies on JME patients, which represents a specific patient group, are very limited. Our study did not show any finding within echocardiographic and HRV parameters suggesting cardiac involvement or autonomic dysfunction in patients with JME who were undergoing treatment.
Accepted for review: 5. 12. 2017
Accepted for print: 10. 10. 2018
Fatma Genç, MD
Department of Neurology
Antalya Training and Research Hospital
Varlık Mahallesi
Kazım Karabekir Cd.
07100 Muratpaşa/Antalya
Turkey
Sources
1. Sperling MR. Sudden unexplained death in epilepsy. Epilepsy Curr 2001; 1(1): 21– 23.
2. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996; 17(3): 354– 381.
3. Pieper SJ, Hammill SC. Heart rate variability: technique and investigational applications in cardiovascular medicine. Mayo Clin Proc 1995 : 70(10); 955– 964. doi: 10.1016/ S0025-6196(11)64374-7.
4. Yi Gang, Malik M. Heart rate variability in general medicine. Indian Pacing Electrophysiol J 2003; 3(1): 34– 40.
5. Bilgi M, Yerdelen D, Cölkesen Y et al. Evaluation of left ventricular diastolic function by tissue Doppler imaging in patients with newly diagnosed and untreated primary generalized epilepsy. Seizure 2013; 22(7): 537– 541. doi: 10.1016/ j.seizure.2013.03.015.
6. ILAE Commission on Classification and Terminology. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989; 30(4): 389– 399.
7. Walczak TS, Leppik IE, D’Amelio M et al. Incidence and risk factors in sudden unexpected death in epilepsy: a prospective cohort study. Neurology 2001; 56(4): 519– 525.
8. DeGiorgio CM, Miller P, Meymandi S et al. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory. Epilepsy Behav 2010; 19 : 78– 81. doi: 10. 1016/ j.yebeh.2010.06.011.
9. Sahn DJ, DeMaria A, Kisslo J et al. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978; 58(6): 1072– 1083.
10. Nagueh SF, Mikati I, Kopelen HA et al. Doppler estimation of left ventricular filling pressure in sinus tachycardia. a new application of tissue doppler imaging. Circulation 1998; 98(16): 1644– 1650.
11. Nagueh SF, Appleton CP, Gillebert TC et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009; 10(2): 165– 193. doi: 10.1093/ ejechocard/ jep007.
12. Teichholz LE, Kreulen T, Herman MV et al. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol 1976; 37(1): 7– 11.
13. Verrier RL, Harper R, Hobson JA. Cardiovascular physiology: central and autonomic regulation. In: Kryger MH, Roth T, Dement WC (eds). Principles and practice of sleep medicine. 5th ed. Philadelphia: WB Saunders Company 2000 : 179– 192.
14. Montano N, Porta A, Cogliati C et al. Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neurosci Biobehav Rev 2009; 33(2): 71– 80. doi: 10.1016/ j.neubiorev. 2008.07.006.
15. Tomson T, Ericson M, Ihrman C et al. Heart rate variability in patients with epilepsy. Epilepsy Res 1998; 30(1): 77– 83.
16. Lotufo PA, Valiengo L, Bensenor IM et al. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia 2012; 53(2): 272– 282. doi: 10.1111/ j.1528-1167.2011.03361.x.
17. Hesdorffer DC, Tomson T, Benn E et al. Do antiepileptic drugs or generalized tonic– clonic seizure frequency increase SUDEP risk? A combined analysis. Epilepsia 2012; 53(2): 249– 252. doi: 10.1111/ j.1528-1167.2011.03354.x.
18. Hallioglu O, Okuyaz C, Mert E et al. Effects of anti-epileptic drug therapy on heart rate variability in children with epilepsy. Epilepsy Res 2008; 79(1): 49– 54. doi: 10.1016/ j.eplepsyres.2007.12.020.
19. Nayak C, Sinha S, Nagappa M et al. Lack of heart rate variability during apnea in patients with juvenile myoclonic epilepsy (JME). Sleep Breath 201; 19(4): 1175– 1183. doi: 10.1007/ s11325-015-1133-y.
20. Ryvlin P, Nashef L, Lhatoo SD et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013; 12(10): 966– 977. doi: 10.1016/ S1474-4422(13)70214-X.
21. Natelson BH, Suarev RV, Terrence CF et al. Patients with epilepsy who die suddenly have cardiac disease. Arch Neurol 1998; 55(6): 857– 860.
22. Woodruff BK, Britton JW, Tigaran S et al. Cardiac troponin levels following monitored epileptic seizures. Neurology 2003; 60(10): 1690– 1692.
23. Tigaran S, Molgaard H, McClelland R et al. Evidence of cardiac ischemia during seizures in drug refractory epilepsy patients. Neurology 2003; 60(3): 492– 495.
24. Alehan F, Erol I, Cemil T et al. Elevated CK-MB massand plasma brain-type natriuretic peptide concentrations following convulsive seizures in children and adolescents: possible evidence of subtle cardiac dysfunction. Epilepsia 2009; 50(4): 750– 760. doi: 10.1111/ j.1528-1167.2008.01793.x.
25. Akashi YJ, Goldstein DS, Barbaro G et al. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008; 118(25): 2754– 2762. doi: 10.1161/ CIRCULATIONAHA.108.767012.
26. Chin PS, Branch KR, Becker KJ. Postictal neurogenic stunned myocardium. Neurology 2005; 64(11): 1977– 1978. doi: 10.1212/ 01.WNL.0000163858.77494.7A.
27. Lemke DM, Hussain SI, Wolfe TJ et al. Takotsubo cardiomyopathy associated with seizures. Neurocrit Care 2008; 9(1): 112– 117. doi: 10.1007/ s12028-008-9075-x.
28. Legriel S, Bruneel F, Dalle L et al. Recurrent Takotsubo cardiomyopathy triggered by convulsive status epilepticus. Neurocrit Care 2008; 9(1): 118– 121. doi: 10.1007/ s12028-008-9075-x.
29. Ramadan MM, El-Shahat N, Omar AA et al. Interictal electrocardiographic and echocardiographic changes in patients with generalized tonic-clonic seizures. Int Heart J 2013; 54(3): 171– 175.
30. Wu CC, Lee WS, Yu WC et al. Impact of left ventricular function on the pulmonary vein Doppler spectrum: nonsimultaneous assessment with load insensitive indices. Echocardiography 2003; 20(1): 9– 18.
31. Kuecherer HF, Muhiudeen IA, Kusumoto FM et al. Estimation of mean left atrial pressure from transesophageal pulsed Doppler echocardiography of pulmonary venous flow. Circulation 1990; 82(4): 1127– 1139.
32. Saetre E, Abdelnoor M, Amlie JP et al. Cardiac function and antiepileptic drug treatment in the elderly: a comparison between lamotrigine and sustained-release carbamazepine. Epilepsia 2009; 50(8): 1841– 1849. doi: 10.1111/ j.1528-1167.2009.02069.x.
33. Drake ME, Reider CR, Kay A. Electrocardiography in epilepsy patients without cardiac symptoms. Seizure 1993; 2(1): 63– 65.
34. Teh HS, Tan HJ, Loo CY et al. Short QTc in epilepsy patients without cardiac symptoms. Med J Malaysia 2007; 62(2): 104– 108.
35. Novak JL, Miller PR, Markovic D et al. Risk assessment for sudden death in epilepsy: the SUDEP-7 ınventory. Front Neurol 2015; 6(252): 1– 5. doi: 10.3389/ fneur. 2015.00252.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2018 Issue 6
-
All articles in this issue
- Diagnostics, symptomatology and findings in diseases and disorders of the autonomic nervous system in neurology
- Patients with extensive early changes (ASPECTS < 5) – recanalization YES
- Patients with extensive early changes (ASPECTS < 5) – recanalization NO
-
Pacient s rozsiahlymi skorými zmenami (ASPECTS < 5) – rekanalizácia
Komentár ku kontroverziám - Pragnancy and multiple sclerosis from a neurologist’s point of view
- Quality of life of caregivers of patients with progressive neurological disease
- New-onset refractory status epilepticus and considered spectrum disorders (NORSE/ FIRES)
- The efficacy of cochlear implantation in adult patients with profound hearing loss
- Clinical results of cervical discectomy and fusion with anchored cage – prospective study with a 24-month follow-up
- A comparison of mini-invasive percutaneous versus classic open pedicle screw fixation of thoracolumbar fractures – retrospective analysis
- Dural reconstruction with usage of xenogenic biomaterial
- Meningococcal meningitis with Chiari malformation (type I)
- Comment to the article N. Dahmardeh et al. Fingolimod attenuates harmaline-induced passive avoidance memory and motor impairments in a rat model of essential tremor
- Evaluation of systolic and diastolic cardiac functions and heart rate variability in patients with juvenile myoclonic epilepsy
- Reconstruction of the anterior skull base with free muscle flap after iatrogenic injury
- A Bulgarian family with epileptic seizures as a first manifestation of familial cerebral cavernous malformations
- Solitary cerebellar metastasis of uterine cervical carcinoma
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Diagnostics, symptomatology and findings in diseases and disorders of the autonomic nervous system in neurology
- New-onset refractory status epilepticus and considered spectrum disorders (NORSE/ FIRES)
- Clinical results of cervical discectomy and fusion with anchored cage – prospective study with a 24-month follow-up
- Pragnancy and multiple sclerosis from a neurologist’s point of view
