Cognitive function of patients receiving whole brain radiotherapy for brain metastases from lung cancer and guidance strategies based on intelligent software
Kognitivní funkce pacientů při celomozkovém ozařování pro mozkové metastázy karcinomu plic a strategie řízení radioterapie pomocí inteligentního softwaru
Cíl: Cílem této studie bylo sledování kognitivních funkcí u pacientů při celomozkovém ozařování (whole brain radiotherapy; WBRT) pro mozkové metastázy (brain metastases; BM) karcinomu plic a analýza strategií řízení radioterapie pomocí inteligentního softwaru. Metody: Byla provedena retrospektivní analýza klinických dat 146 pacientů s BM karcinomu plic, přičemž pacienti byli rozděleni do skupiny A (n = 95, pacienti s neurologickými symptomy) a skupiny B (n = 51, pacienti bez neurologických symptomů). Byla provedena multivariační logistická regresní analýza s cílem prozkoumat rizikové faktory pro zhoršení kognitivních funkcí a byl zkonstruován a zhodnocen predikční model. Výsledky: Po WBRT se ve skupině A výrazně zvýšilo skóre tzv. krátkého testu kognitivních funkcí (Mini-Mental State Examination; MMSE) (p < 0,05), které bylo nejvyšší ve 4. měsíci a pak došlo k mírnému poklesu. Naproti tomu ve skupině B došlo po WBRT ke snížení skóre, přičemž nejnižší bylo ve 4. měsíci a pak došlo k jeho zvýšení. Logistická analýza ukázala, že nezávislými rizikovými faktory pro zhoršení kognitivních funkcí byla chemoterapie podávaná ve ≥ 3 cyklech se simultánním integrovaným boostem, ozáření v dávce > 30 Gy a absence hipokampus šetřící techniky ozařování (p < 0,05). Závěr: Technika WBRT má na BM karcinomu plic zřetelné terapeutické účinky, ale ovlivňuje kognitivní funkce, což je nejvíc patrné ve 4. měsíci léčby.
Klíčová slova:
kognitivní funkce – karcinom plic – mozkové metastazy – celomozkové ozáření
Authors:
Yueyue Chen *; L. Zhang *; J.-F. Huang; W. Jiang; M.-Q. Xu
Authors‘ workplace:
Affiliated Hospital of Jiangnan University, Jiangsu Province, China
; The two authors contributed equally to this study.
*
Published in:
Cesk Slov Neurol N 2022; 85(6): 489-495
Category:
Original Paper
doi:
https://doi.org/10.48095/cccsnn2022489
Overview
Aim: The aim of this study was to investigate cognitive functions of patients receiving whole brain radiotherapy (WBRT) for brain metastases (BM) from lung cancer, and to analyze guidance strategies based on intelligent software. Methods: The clinical data of 146 patients with BM from lung cancer were collected for retrospective analysis, and they were assigned to group A (N = 95, presence of neurological symptoms) and group B (N = 51, absence of neurological symptoms). Multivariate logistic regression analysis was employed to explore the risk factors for cognitive decline, and a prediction model was constructed and evaluated. Results: After WBRT, the Mini-Mental State Examination (MMSE) score of group A increased significantly (P < 0.05), the highest was in the 4th month and then decreased slightly. In contrast, the MMSE score of group B was lowered after WBRT, the lowest was in the 4th month and rose thereafter. Logistic analysis indicated that chemotherapy ≥ 3 cycles, simultaneous integrated boost, irradiation dose > 30 Gy and absence of hippocampal avoidance were independent risk factors for cognitive decline (P < 0.05). Conclusion: WBRT has obvious therapeutic effects on BM from lung cancer, but it influences the cognitive functions, which is the most noticeable in the 4th month.
Keywords:
lung cancer – cognitive function – brain metastases – whole brain radiotherapy
Introduction
Brain metastases (BM) mainly present with intracranial hypertension and localized headache at early clinical stage [1,2]. If these patients develop diffuse headache, concomitant symptoms (including vomiting, cognitive disorders, fatigue, or even hemiplegia and sensory loss) may occur. In the medical field, BM from malignancies have always been considered an end-stage indication [3]. As therapeutic technology continuously advances, radiotherapy, new chemotherapeutic agents and targeted drugs have achieved better curative effects. Statistics show that the proportion of BM from lung cancer accounts for about 70–80% of all patients with BM [4]. It has been reported that the median survival time of patients with BM from lung cancer is no more than 3 months if they receive symptomatic and supportive treatment only [5].
Currently, whole brain radiotherapy (WBRT) is the standard method commonly used to treat BM [6]. Relevant studies have shown that after WBRT, the median survival time of patients with < 3 BM from lung cancer and those with > 3 BM can be extended to 10 and 4 months, respectively. It has been that WBRT possibly reduces cognitive functions and quality of life, which are also associated with patients‘ conditions and lesions [7]. However, relevant research has rarely been reported in China. At present, intelligent software is available to construct prediction models on the basis of numerous clinical information and data [8], which is superior to traditional statistical methods in predictive accuracy and clinical value.
Hence, this study aimed to explore the cognitive functions of patients receiving WBRT for BM from lung cancer and analyze guidance strategies based on intelligent software, so as to provide references for clinical treatment and diminish the impact of WBRT on patients‘ cognitive functions.
Patients and methods
General data
A total of 146 patients with BM from lung cancer admitted to the Department of Radiotherapy in our hospital from January 2019 to February 2021 were selected as research subjects. According to the presence or absence of neurological symptoms (including memory loss, lethargy, headache, dizziness, sensory or motor abnormalities, epileptic seizures, cognitive or speech disorders), these patients were categorized into group A (N = 95, presence of neurological symptoms) and group B (N = 51, absence of neurological symptoms). In group A, there were 54 males and 41 females aged 35–80 (58.41 ± 9.30) years, of whom 54 patients had received < 12 years of education (high school and below) and 45 patients had received ≥ 12 years of education (undergraduate and above). In group B, there were 26 males and 25 females aged 34–78 (56.37 ± 8.82) years, of whom 22 patients had received < 12 years of education and 29 patients had received ≥ 12 years of education.
Inclusion criteria were as follows:
1) patients definitely diagnosed with BM from lung cancer by MRI for the first time;
2) those who received WBRT and had no history of other brain radiotherapy or surgery;
3) those who had no severe diseases and could cooperate with radiotherapy;
4) those who were able to complete the questionnaire either on their own or with the aid of others.
Exclusion criteria were as follows:
1) patients with dementia;
2) those with cognitive decline induced by other causes;
3) those with an expected survival time of < 3 months.
General data collection
Clinical data were gathered from each eligible subject, including 1) clinical efficacy of WBRT [5]; 2) Mini-Mental State Examination (MMSE) scores before and after WBRT [5]; 3) age, sex, underlying diseases affecting cognitive functions, lesion volume, lesion location, chemotherapy cycle, simultaneous integrated boost, irradiation dose, years of education, number of intracranial metastases, hippocampal avoidance, and pathology of the primary lesion.
Whole brain radiotherapy
Chemotherapy was administered during WBRT. WBRT was applied using the PRIMUS-H linear accelerator (Siemens, Munich, Germany) with an X-ray beam energy of 6 MV. Specifically, the patient was in a supine position with hands on both sides of the body and the head was fixed with a thermoplastic mask. CT simulation system was used for positioning (upper boundary: cranial vault; lower boundary: lower edge of C1). According to CT images, the target region and the hippocampal region (hippocampus and 5 mm region around the hippocampus) were delineated layer by layer. The irradiation dose was set as DT30 Gy (3.0 Gy/time, 5×/week) or DT40 Gy (2.0 Gy/time, 5×/week).
Statistical analysis
SPSS 23.0 (IBM, Armonk, NY, USA) software was used for statistical analysis. Measurement data conforming to normal distribution were expressed as mean ± standard deviation (c ± s) and analyzed by two independent sample t-tests for comparison between the groups. Non-normally distributed measurement data were expressed as the median [M (Q1, Q3) ] and analyzed using the Mann-Whitney U test for comparison between groups. Enumeration data were expressed as frequency (%) and analyzed using the c2 test for comparison between groups. Besides, logistic regression analysis was used to analyze independent risk factors for cognitive decline. A back propagation (BP) neural network model was constructed, and repeated cross-validation was employed to verify the number of hidden layer nodes. The predictive accuracy and validity of the model were evaluated by calibration curve and clinical decision curve. The significance level was set as a = 0.05.
Results
Clinical efficacy of WBRT
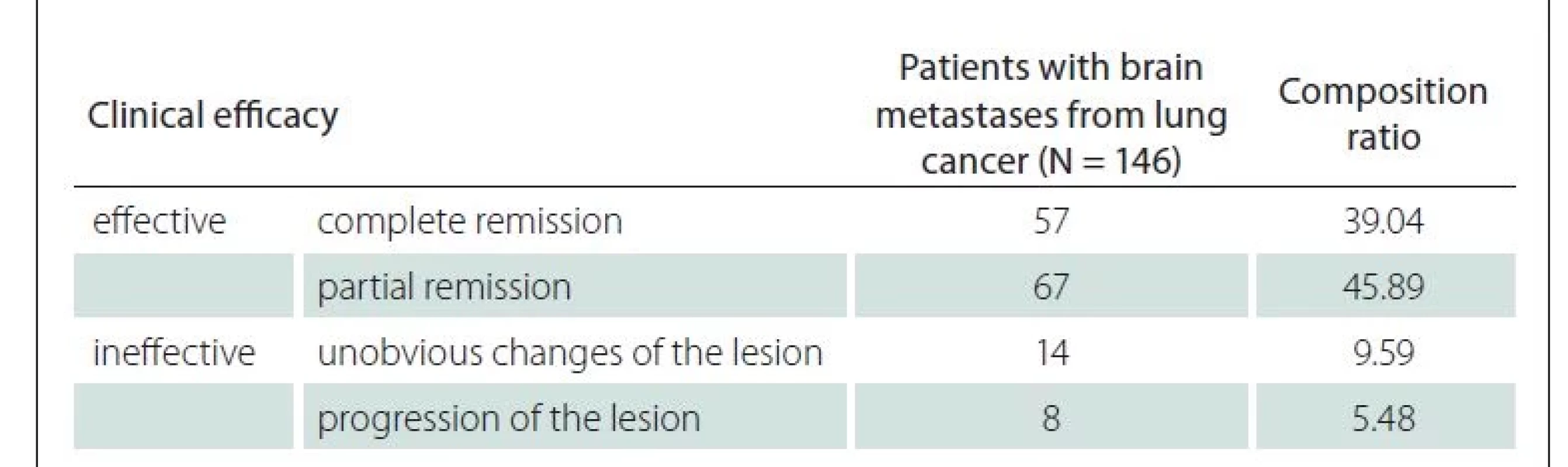
Among the 146 patients, WBRT was clinically effective in 124 cases (84.93%) (complete and partial remission) and ineffective in 22 cases (15.07%) (unobvious changes and progression of the lesion) (Tab. 1).
MMSE scores before and after WBRT
Before WBRT, group A showed a lower MMSE score than group B (P < 0.05). After WBRT, as the treatment time went by (1–6 months), the MMSE score in group A significantly increased (P < 0.05), and it was the highest in the 4th month and then declined slightly. In contrast, the MMSE score was lower in group B, and it was the lowest in the 4th month and rose thereafter (Tab. 2).
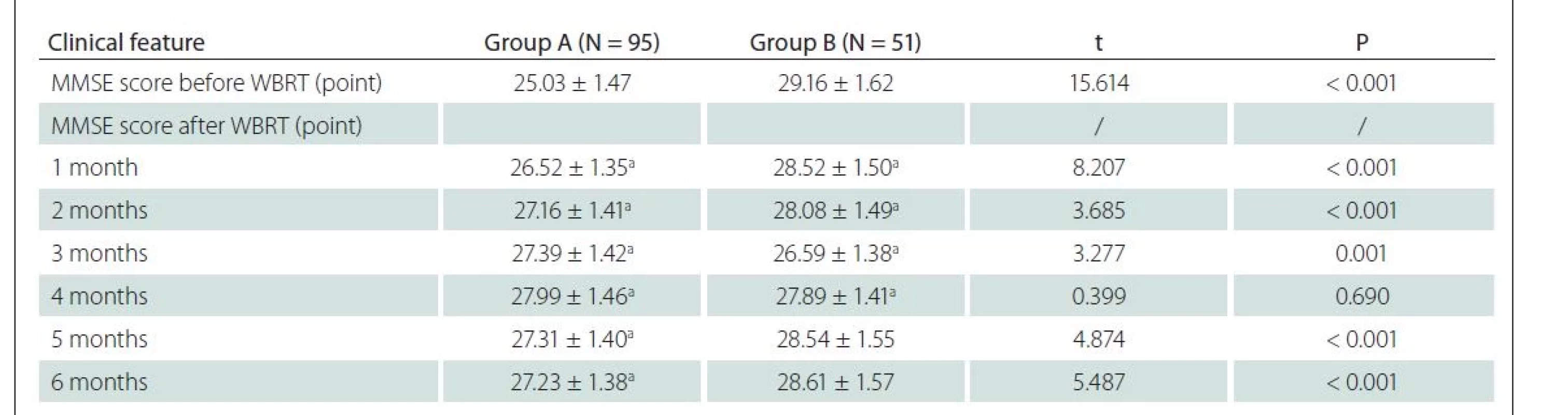
MMSE – Mini Mental State Examination; N – number; WBRT – whole brain radiotherapy
Clinical data of patients
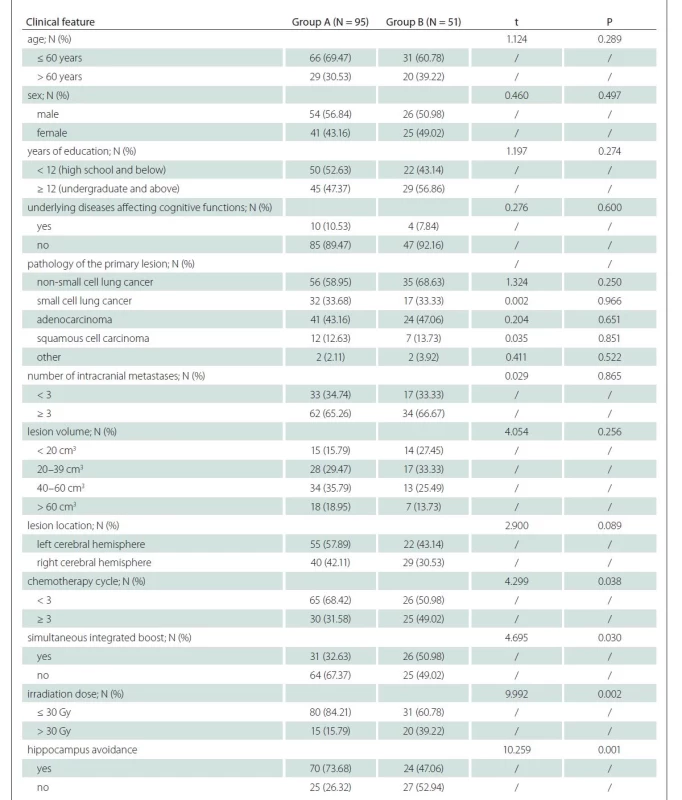
Clinical data of patients were compared between the two groups to explore the influencing factors for cognitive decline after WBRT (< 4 months). Compared with group A, the proportions of patients with chemotherapy ≥ 3 cycles, simultaneous integrated boost, irradiation dose > 30 Gy and absence of hippocampal avoidance were statistically significantly higher in group B (P < 0.05). The differences in remaining clinical data were not statistically significantly different between the two groups (P > 0.05) (Tab. 3).
Results of multivariate logistic regression analysis
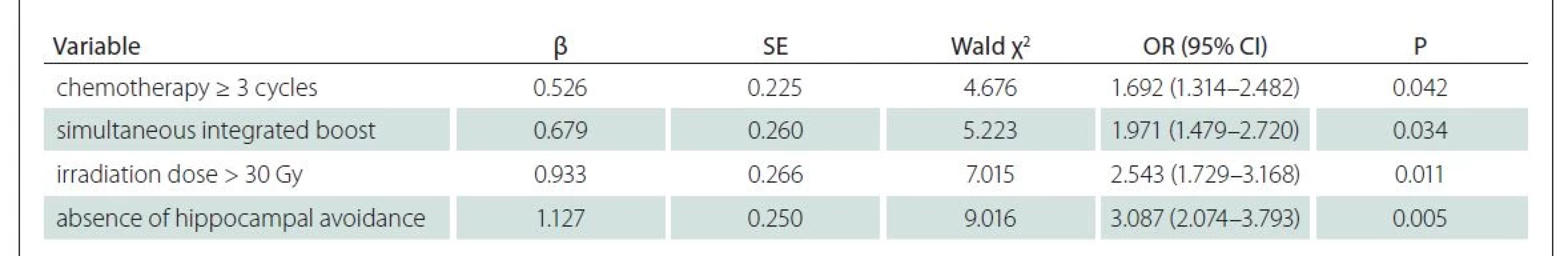
Multivariate logistic regression analysis was conducted with cognitive decline as a dependent variable and indicators with statistically significant difference between the two groups in Tab. 3 (P < 0.05) as independent variables. The results revealed that chemotherapy ≥ 3 cycles, simultaneous integrated boost, irradiation dose > 30 Gy and absence of hippocampal avoidance were independent risk factors for cognitive decline (P < 0.05) (Tab. 4 and Fig. 1).
MMSE – Mini Mental State Examination; WBRT – whole brain radiotherapy
Obr. 1. Graf faktorů ovlivňujících pokles kognitivních funkcí.
MMSE – Mini Mental State Examination; WBRT – ozařování celého mozku

Construction of BP neural network model
The number of hidden layer nodes was determined via repeated cross-validation. The root mean square error of cross-validation was the smallest when the number of hidden layer nodes was 3 (Fig. 2). The 4 influencing factors for patients‘ cognitive decline were incorporated into the BP neural network model, which was the input layer, and cognitive decline status in patients was the output layer. Based on these, a BP neural network model was constructed (Fig. 3).
CI – confidence interval; OR – odds ratio
Obr. 2. Určení počtu uzlů skrytých vrstev pomocí křížové validace.
CI – interval spolehlivosti; OR – odds ratio

Obr. 3. Model neuronové sítě mozkových metastáz.

Model evaluation
The calibration curve and clinical decision curve were utilized to evaluate the accuracy and validity of the BP neural network model in predicting cognitive decline in patients. The calibration curve illustrated that the predicted probability of the model fitted well with the actual probability, and Hosmer-Lemeshow goodness-of-fit test showed no statistically significant difference (c2 = 1.859; P = 0.126), suggesting that the accuracy of the model was relatively high (Fig. 4). The clinical decision curve denoted that when the threshold probability of the model was within the range of 0.01–0.84, the net benefit rate was > 0, indicating that the validity of the model was relatively favorable (Fig. 5).
BP – brain metastases
Obr. 4. Kalibrační křivka modelu.
BP – mozkové metastázy

BP – brain metastases
Obr. 5. Klinická rozhodovací křivka modelu.
BP – mozkové metastázy

Discussion
Lung, liver, brain, bone and adrenal gland are the five organs that easily develop metastases from malignancies. Currently, it has been proven that BM are among the most common intracranial tumors [9]. Intracranial metastases from malignant tumors are mainly transmitted through blood, lymph and direct invasion, of which blood transmission is the most common. The route and location of metastasis are relevant to the primary lesion site. For instance, lung cancer and breast cancer are prone to blood metastases, while gastrointestinal cancer is prone to lymph metastases. Statistics show that about 20–40% of malignant tumors may be complicated by BM as the illness progresses [10,11]. Particularly, over 90% of lung adenocarcinoma metastasizes to cerebral tissue, mostly presenting as multiple lesions at the gray-white matter border and distribution area of cerebral arteries. In general, the presence of BM from tumors indicates that the disease has developed into advanced stage, i.e., if not treated promptly and effectively, the illness will be exacerbated rapidly, and the expected median survival time is about 3 months. At present, BM are mainly treated by controlling tumor growth, alleviating pain and protecting cognitive functions, so as to prolong survival time and improve quality of life. As a dominant treatment modality, WBRT has achieved favorable efficacy in both primary lesions and multiple BM [12]. Nonetheless, long-term radiotherapy possibly causes damage to cerebral white matter, vascular endothelial cells and cognitive functions, tremendously affecting patients‘ quality of life.
In this study, the results denoted that WBRT had a better clinical efficacy, with a total effective rate of 84.93%. In respect of cognitive functions, group A had a markedly increased MMSE score after WBRT, and it was the highest in the 4th month and then declined slightly, whereas the MMSE score in group B was decreased after WBRT, it was the lowest in the 4th month and rose thereafter. The findings suggested that WBRT influenced cognitive functions of BM patients to a certain extent, i.e., it can improve the cognitive functions of patients with neurological symptoms before WBRT, but causes the decline of cognitive functions of patients without neurological symptoms before treatment. The reasons are as follows. Since patient‘s condition is effectively controlled and even the lesions are diminished after WBRT, the cognitive functions of patients, which are also affected by BM, may improve after treatment. However, in the absence of neurological symptoms, radiation can directly or indirectly cause chronic cerebral cortical and structural damage [13] leading to cognitive impairment.
Multivariate logistic regression analysis showed that chemotherapy ≥ 3 cycles, simultaneous integrated boost, irradiation dose > 30 Gy and absence of hippocampal avoidance were independent risk factors for cognitive decline after WBRT. Specifically, brain regions are sensitive to external stimuli, particularly radiation, so brain tissue and cognitive impairment may be induced by long-term WBRT and simultaneous integrated boost or irradiation dose > 30 Gy. In addition, the hippocampus is located between the cerebral thalamus and the medial temporal lobe and is a component of the limbic system. Hippocampus is dominantly responsible for storage, conversion and orientation of short-term memory, which is relevant to higher nervous activity such as emotions, learning and memory, and the cells in the subgranular zone of the hippocampus are sensitive to radiation. Warrington et al [14] reported that post-WBRT cognitive dysfunction may be related to cell damage in the subgranular zone of the hippocampus. Thus, hippocampal-avoidance WBRT is capable of effectively relieving cognitive decline.
In the field of artificial intelligence, intelligent assistance software is a relatively novel method that can integrate and analyze data in the massive datasets obtained from clinical practice using automatically improved computer algorithms, thereby providing new insights and approaches for precise clinical prediction [15]. In this study, therefore, the relevant influencing factors for cognitive decline were incorporated into multivariate logistic regression analysis, followed by construction of BP neural network prediction model. The number of hidden layer nodes in the BP neural network model was 3, and the calibration curve and clinical decision curve illustrated that the model had favorable predictive accuracy and validity. This method not only avoids the drawbacks of traditional methods, such as excessive data, complex operation and limitations, but also provides references for clinical diagnosis and risk prediction.
In summary, chemotherapy ≥ 3 cycles, simultaneous integrated boost, irradiation dose > 30 Gy and absence of hippocampal avoidance are independent risk factors for cognitive decline in patients with BM from lung cancer receiving WBRT. The BP neural network model has favorable predictive accuracy and validity. Based on these, chemotherapy cycle, irradiation dose and hippocampal avoidance should be taken into account according to the patient‘s situation when WBRT is utilized by clinicians for treatment of BM, to strengthen the protection of patients‘ brain regions and cognitive functions.
Ethical principles
The entire study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 2004 and 2008). This study was approved by the Ethics Committee of our hospital (no: LS2019056, date: 11. 1. 2019), and informed consent was obtained from all patients or their family members.
Financial support
This study was financially supported by The General Project of the Scientific Research Project of Wuxi Municipal Health Commission (No. MS201928).
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
Li Zhang
Affiliated Hospital of Jiangnan
University
Wuxi 214061
Jiangsu Province
China
e-mail: zhangliahju@dh-edu.cn
Accepted for review: 31. 5. 2022
Accepted for print: 8. 11. 2022
Sources
1. Haim O, Abramov S, Shofty B et al. Predicting EGFR mutation status by a deep learning approach in patients with non-small cell lung cancer brain metastases. J Neurooncol 2022; 157 (1): 63–69. doi: 10.1007/s11060-022-03946-4.
2. Jiang Z, Wang B, Han X et al. Multimodality MRI-based radiomics approach to predict the posttreatment response of lung cancer brain metastases to gamma knife radiosurgery. Eur Radiol 2022; 32 (4): 2266–2276. doi: 10.1007/s00330-021-08368-w.
3. Niranjan A, Monaco E, Flickinger J et al. Guidelines for multiple brain metastases radiosurgery. Prog Neurol Surg 2019; 34 : 100–109. doi: 10.1159/000493 055.
4. Hanke B, Jünger ST, Kirches E et al. Frequency of actionable molecular drivers in lung cancer patients with precocious brain metastases. Clin Neurol Neurosurg 2021; 208 : 106841. doi: 10.1016/j.clineuro.2021.106841.
5. Ernani V, Stinchcombe TE. Management of brain metastases in non-small-cell lung cancer. J Oncol Pract 2019; 15 (11): 563–570. doi: 10.1200/JOP.19.00357.
6. Mulvenna P, Nankivell M, Barton R et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016; 388 (10055): 2004–2014. doi: 10.1016/S0140-6736 (16) 30825-X.
7. Chang EL, Wefel JS, Hess KR et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009; 10 (11): 1037–1044. doi: 10.1016/S1470-2045 (09) 70263-3.
8. Lei G, Wang G, Zhang C et al. Using machine learning to predict acute kidney injury after aortic arch surgery. J Cardiothorac Vasc Anesth 2020; 34 (12): 3321–3328. doi: 10.1053/j.jvca.2020.06.007.
9. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol 2005; 75 (1): 5–14. doi: 10.1007/s11060-004-8093-6.
10. Soffietti R, Abacioglu U, Baumert B et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol 2017; 19 (2): 162–174. doi: 10.1093/neuonc/now241.
11. Noh T, Walbert T. Brain metastasis: clinical manifestations, symptom management, and palliative care. Handb Clin Neurol 2018; 149 : 75–88. doi: 10.1016/B978-0-12-811161-1.00006-2.
12. Kim JS, Kim K, Jung W et al. New brain metastases after whole-brain radiotherapy of initial brain metastases in breast cancer patients: the significance of molecular subtypes (KROG 16-12). Breast Cancer Res Treat 2021; 186 (2): 453–462. doi: 10.1007/s10549-020-06043-0.
13. Garsa A, Jang JK, Baxi S et al. Radiation therapy for brain metastases: a systematic review. Pract Radiat Oncol 2021; 11 (5): 354–365. doi: 10.1016/j.prro.2021.04.002.
14. Warrington JP, Csiszar A, Johnson DA et al. Cerebral microvascular rarefaction induced by whole brain radiation is reversible by systemic hypoxia in mice. Am J Physiol Heart Circ Physiol 2011; 300 (3): H736–744. doi: 10.1152/ajpheart.01024.2010.
15. Chen M, Decary M. Artificial intelligence in healthcare: an essential guide for health leaders. Healthc Manage Forum 2020; 33 (1): 10–18. doi: 10.1177/0840470419873123.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2022 Issue 6
-
All articles in this issue
- Limb girdle muscular dystrophies
- Progress in knowledge of migraine pathophysiology
- Basic principles of anaesthetic care for intraoperative transcranial motor evoked potentials monitoring
- New pharmacological options in the treatment of Alzheimer‘s disease
- The role of scoring systems in treatment indication of meningiomas in elderly patients
- Validation study and introduction of the new TEPO sentence comprehension test for children aged 3–8 years
- Clonal hematopoiesis of indeterminate potential in ischemic stroke – study protocol
- Chronic immune sensory polyradiculopathy associated with monoclonal gammopathy of undetermined significance
- Borderline concentrations of a cerebrospinal fluid triplet of tau proteins and beta-amyloid 42 in the diagnosis of Alzheimer‘s disease and other neurodegenerative dementias
- Guidelines for developmental dysphasia – version 2022
- Potential of the projective Colour Association Method to reflect physiological responses to stimuli with a different emotional charge (PARC study) – a study protocol
- Cognitive function of patients receiving whole brain radiotherapy for brain metastases from lung cancer and guidance strategies based on intelligent software
- Extrapontine central myelinolysis with extrapyramidal symptoms in a 14-year-old boy with COVID-19 disease-related PIMS-TS
- Middle ear myoclonus as a cause of objective tinnitus
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Guidelines for developmental dysphasia – version 2022
- Validation study and introduction of the new TEPO sentence comprehension test for children aged 3–8 years
- New pharmacological options in the treatment of Alzheimer‘s disease
- Limb girdle muscular dystrophies
