The Changing Microbiological Pattern in Patients with Confirmed Bacterial Meningitis after Post-craniotomy Surgery
Změna mikrobiologickýho vzorku u pacientů s potvrzenou bakteriální meningitidou po postkraniotomiální operaci
Meningitida po kraniotomii(PCM) představuje důležitou výzvu v neurochirurgii. Tato práce popisuje diagnostikovanou PCM u 22 z 4 392 pacientů s provedenou kraniotomií v intervalu mezi 1. lednem 2008 a 31. prosincem 2012. U sedmi pacientů s meningitidou způsobenou bakterií Acinetobacter baumannii a u 15 pacientů s neprokázanou Acinetobacter baumannii meningitidou byly signifikantní rozdíly ve výskytu traumatu hlavy (OR = 5,873, 95% CI 1,575–17,511; p = 0,004), a lokalizací infekce mimo centrální nervový systém (OR = 0,872, 95% CI 0,665–1,621; p = 0,003). Naše sdělení nabízí lékařům aktuální pohled na měnící se vzorec infekčních agens u PCM.
Klíčová slova:
meningitida – neurochirurgie – Acinetobacter baumannii – Staphylococcus aureus – poranění hlavy
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors:
Ch. Chang-Hua 1; Y. Hua-Cheng 2; Ch. Yu-Jun 3; L. Chih-Wei 4
Authors place of work:
Changhua Christian Hospital, Changhua, Taiwan, Division of Infectious Disease, Department of Internal Medicine
1; Changhua Christian Hospital, Changhua, Taiwan, Division of Neurosurgery, Department of Surgery
2; Changhua Christian Hospital, Changhua, Taiwan, Laboratory of Epidemiology and Biostatistics
3; Changhua Christian Hospital, Changhua, Taiwan, Department of Medical Imaging
4
Published in the journal:
Cesk Slov Neurol N 2017; 80/113(1): 80-83
Category:
Krátké sdělení
Summary
Post-craniotomy meningitis (PCM) is an important challenge in neurosurgery. In this study, PCM was diagnosed in 22 of the 4,392 post-craniotomy patients between January 1, 2008 and December 31, 2012. There were significant statistical differences between the seven patients with Acinetobacter baumannii meningitis and 15 patients with non-Acinetobacter baumannii meningitis with respect to the history traumatic head injury (OR = 5.873, 95% CI 1.575–17.511; p = 0.004) and infected sites other than the central nervous system (OR = 0.872, 95% CI 0.665–1.621; p = 0.003). We provide the most up-to-date insight to physicians on the changing pattern of infectious agents in PCM.
Key words:
meningitis – neurosurgery – Acinetobacter baumannii – Staphylococcus aureus – head trauma
Chinese summary - 摘要
确认开颅手术后细菌性脑膜炎患者发生微生物学菌的改变开颅手术后发生的脑膜炎(简称术后脑膜炎),是神经外科病患中的重要议题。 本研究中,收集2008年1月1日至2012年12月31日期间,在本院接受开颅手术的4,392例患者,并且确定诊断出术后脑膜炎,总共有22例,进行分析。全部22例术后脑膜炎病患分成两群,其中七名鲍曼不动杆菌脑膜炎患者,和另外的15名非鲍氏不动杆菌脑膜炎的患者之间,进行比较,发现术后脑膜炎中,属于发生术后脑膜炎以前有遭遇过外伤的族群中,有较多病患会出现鲍曼不动杆菌的趋势(OR = 5.873,95%CI 1.575-17.511; p = 0.004)此外,这些术后脑膜炎中,如果在中枢神经系统以外,还有其他的感染部位,,有较少会出现鲍曼不动杆菌的趋势(OR = 0.872,95%CI 0.665-1.621; p = 0.003)。 本研究为提供临床医师,在照护术后脑膜炎时候,需要及时地注意地区性术后脑膜炎发生致病微生物学菌相的改变,为病患选用更适当的抗生素,来的治疗病患。
Introduction
Post-craniotomy meningitis (PCM) results in severe morbidity and mortality [1,2]. The rates of PCM have been reported to vary from 0.3 to 10%, and Staphylococcus aureus (S. aureus) is the major pathogen [2,3]. Some studies revealed that the rate of PCM due to Acinetobacter baumannii complex (A. baumannii) is increasing [1,4–6], and A. baumannii meningitis (ABM) causes high mortality rates [5]. In addition, the rate of PCM due to A. baumannii has become accretive from China, Iran and Turkey [1,4–6]. The limited epidemiological data can be perplexing for physicians, in both making accurate diagnosis and choosing effective antibiotics, hence an urgent clinical need was behind the motivation to perform this study to determine the current pattern of infectious agents of PCM in Changhua Christian Hospital (CCH).
Observation
The study was approved by the ethics committee of CCH (CCH IRB No. 131118). CCH is a 1,800-bed tertiary referral medical center, and more than 1,500 craniotomies are performed in CCH every year. This retrospective study included 22 patients who developed post-neurosurgical meningitis among the 4,392 patients who underwent craniotomy procedures between January 1, 2008 and December 31, 2012. Cases of post-craniotomy meningitis were identified from microbiological databases and medical records according to the International Classification of Diseases, ninth edition, clinical modifications (ICD-9-CM) at CCH in central Taiwan. The diagnosis of meningitis was based on the inclusion criteria and included both significant clinical evidence and microbiological confirmation. The definition of surgical site infection (SSI) is according to CDC/NHSN surveillance definitions [7]. The Vitek-2 System (BioMerieux, Marcy l‘Etoile, France) was used to identify the phenotype of isolates. SPSS version 21.0 for Windows (SPSS Inc., Chicago, IL, USA) were used statistical analysis.
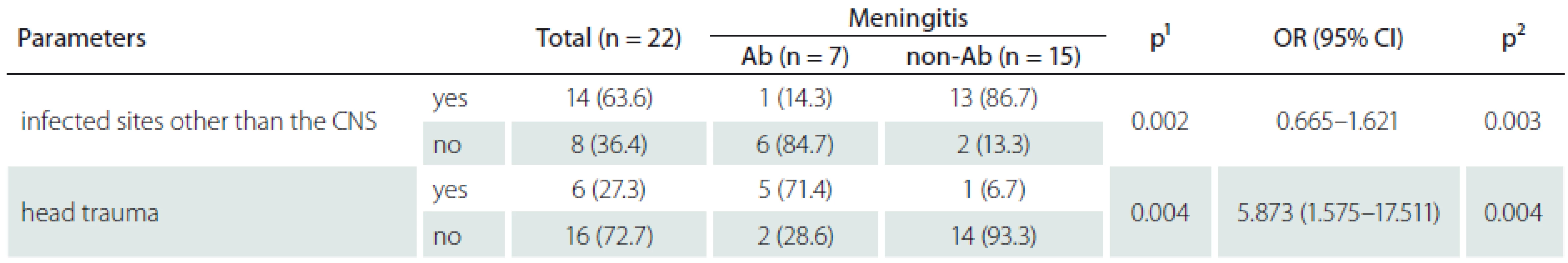
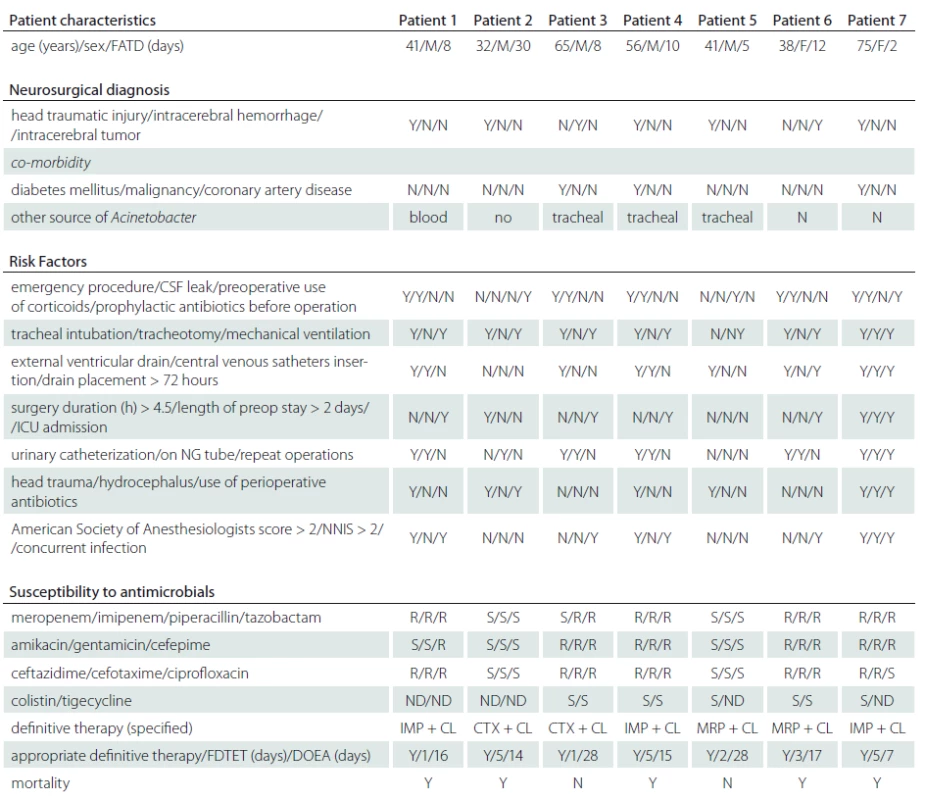
During the study period, PCM was diagnosed in 22 of the 4,392 operated patients. The prevalence of PCM in CCHS was 134.4 ± ± 76.8 per 100,000 patient-years during the study period. The incidence of PCM was 0.5% among the 4,392 operated patients in CCH. The five-year longitudinal analysis of healthcare-associated infections (HAI), SSI, and total number of craniotomies and total number of PCMs during the study period is shown in Graph 1. The key risk factors among the 4,392 patients who underwent post-craniotomy operation and in whom PCM occurred included older age (15/1,821), emergency procedure (16/2,524), CSF leakage (15/1,822), external ventricular drainage (12/1,256), intensive care unit admission (11/1,160), drain placement duration more than 72 hours (13/993), surgery duration more than 4.5 hours (6/312), repeated operations (7/565), head trauma (6/351). The pathogens isolated from the CSF were S. aureus in 8 (36.4%), A. baumannii in 7 (31.8%), P. aeruginosa in 2 (9.1%), K. pneumoniae in 2 (9.1%) , E. cloacae in 1 ( 4.5%), E. coli in 1 (4.5%), and E. faecalis in 1 (4.5%). Of the 22 patients with PCM, 14 (63.6%) had at least one concurrent HAI, and the distribution of these HAI events was SSI in 15 (68.2%), pneumonia in 4 (28.6%), bacteremia in 4 (28.6%), urinary tract infection in 2 (14.3%), and central venous catheter-related infection in 1 (7.1%). Of the 22 patients with PCM, 6 (27.3%) died. There were no significant statistical differences between patients with ABM and non-ABM (Tab. 1), except for head traumatic injury (OR = 5.873, 95% CI 1.575–17.511; p = 0.004), and infected sites other than the central nervous system (CNS) (OR= 0.872, 95% CI = 0.665–1.621; p = 0.003). In this study, we disclosed that patients after traumatic head injury had higher risk of ABM (Graph 1). The clinical information of ABM is listed in Tab. 2.

Discussion
This study showed that ABM emerged in post-traumatic head injury patients with PCM. Many studies showed that non-fermentative gram-negative bacteria (GNB), most commonly Acinetobacter spp., have become important in recent years [4,8,9]. We conducted an evidence-based literature review because ABM has been rarely suggested as a cause of CNS infection [10–14]. Among the 22 PCM patients, PCM was caused by A. baumannii in 5 of 6 patients with traumatic head injury and in 2 of 16 patients without traumatic head injury. Among the 22 PCM patients, PCM was caused by A. baumannii in 6 of 8 patients with only one CNS infection source. A. baumannii and S. aureus were the most commonly isolated agents in PCM and this is in line with the suggested global emergence and increase of A. baumannii colonization and infection, resulting from skin or wound colonization, invasive procedures, sourced from a head traumatic wound or nostrils, and some kind of predominant cluster. In our study, S. aureus was still the most common pathogen in patients with PCM but we emphasize that physicians should also concentrate on A. baumannii when treating PCM patients.
There were no significant statistical differences between meningitis group and non-meningitis group in key risk factors. However, 5 (71.4%) of 7 ABM had a history of traumatic head injury and they died. Our results are similar to those of Baltas’s report that showed 6 of 12 infectious agents being GNB among 860 patients who developed complications after traumatic injury [9,10].We identified eight post-craniotomy S. aureus meningitis and seven post-craniotomy ABMs with 6 of them suffering from traumatic head injury. Hence, we assume that patients after traumatic head injury undergoing craniotomy easily acquire A. baumannii and S. aureus infection.
In conclusion, we found a high rate of PCM due to A. baumannii and S. aureus among patients with traumatic head injury. The changing pattern of infectious agents in PCM over time suggests the need for further studies to provide the most up-to-date insight to physicians.
The present work was partially supported by a grant from the Changhua Christian Hospital (grant 103-CCH-IPR-001 and 104-CCH-IPR-001). The authors thank the staff of the Clinical Microbiology Laboratory, Department of Medical Records, and Department of Computing of Changhua Christian Hospital for case findings. The authors thank prof. Min-Chi Liu for proof reading of this article. This research project would not have been possible without the support of many people. The authors wish to express their gratitude to the staff of the Infection Control Committee, Division of Infectious Diseases, Department of Nursing, Department of Healthcare Quality, and Department of Pharmacology of the Changhua Christian Hospital who were extremely helpful and provided invaluable assistance and support. We thank Fu-Chou Chen serving at the Taichung Veterans General Hospital for reviewing this article.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Chen Chang-Hua, MD
Division of Infectious Disease
Department of Internal Medicine
Changhua Christian Hospital
135 Nanhsiao Street
Changhua 500
Taiwan
e-mail: 76590@cch.org.tw
Accepted for review: 24. 6. 2016
Accepted for print: 18. 8. 2016
Zdroje
1. Chen C, Zhang B, Yu S, et al. The incidence and risk factors of meningitis after major craniotomy in china: a retrospective cohort study. PLoS One 2014;9(7):e101961. doi: 10.1371/ journal.pone.0101961.
2. McClelland S, Hall WA. Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis 2007;45(1):55–9.
3. van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med 2010;362(2):146–54. doi: 10.1056/ NEJMra0804573.
4. Yadegarynia D, Gachkar L, Fatemi A, et al. Changing pattern of infectious agents in postneurosurgical meningitis. Caspian J Intern Med 2014;5(3):170–5.
5. Metan G, Alp E, Aygen B, et al. Carbapenem-resistant acinetobacter baumannii: an emerging threat for patients with post-neurosurgical meningitis. Int J Antimicrob Agents 2007;29(1):112–3.
6. Kourbeti IS, Vakis AF, Ziakas P, et al. Infections in patients undergoing craniotomy: risk factors associated with post-craniotomy meningitis. J Neurosurg 2015;122(5):1113–9. doi: 10.3171/ 2014.8.JNS132557.
7. National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004;32(8):470–85.
8. Howard A, O‘Donoghue M, Feeney A, et al. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence 2012;3(3):243–50. doi: 10.4161/ viru.19700.
9. Baltas I, Tsoulfa S, Sakellariou P, et al. Posttraumatic meningitis: bacteriology, hydrocephalus, and outcome. Neurosurgery 1994;35(3):422–6.
10. Karaiskos I, Galani L, Baziaka F, et al. Successful treatment of extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis with intraventricular colistin after application of a loading dose: a case series. Int J Antimicrob Agents 2013;41 : 480–3. doi: 10.1016/ j.ijantimicag.2013.02.010.
11. Chen CH, Chen YM, Cheng CY. Two cases reports of Acinetobacter baumannii meningitis: an emerging threat for post-neurosurgical patients: case report. J Intern Med Taiwan 2014;25 : 436–45.
12. Fernandez-Viladrich P, Corbella X, Corral L, et al. Successful treatment of ventriculitis due to carbapenem-resistant Acinetobacter baumannii with intraventricular colistin sulfomethate sodium. Clin Infect Dis 1999;28(4):916–7.
13. Khawcharoenporn T, Apisarnthanarak A, Mundy LM.Intrathecal colistin for drug-resistant Acinetobacter baumannii central nervous system infection: a case series and systematic review. Clin Microbiol Infect 2010;16(7): 888–94. doi: 10.1111/ j.1469-0691.2009.03019.x.
14. Cascio A, Conti A, Sinardi L, et al. Post-neurosurgical multidrug-resistant Acinetobacter baumannii meningitis successfully treated with intrathecal colistin. A new case and a systematic review of the literature. Int J Infect Dis 2010;14(7):e572–9. doi: 10.1016/ j.ijid.2009.06.032.
Štítky
Dětská neurologie Neurochirurgie NeurologieČlánek vyšel v časopise
Česká a slovenská neurologie a neurochirurgie

2017 Číslo 1
-
Všechny články tohoto čísla
- Diagnostic Pitfalls of an Atypical Form of Congenital Muscular Dystrophy – Partial Merosin Deficiency – Case Reports
- Extirpation of Colloid Cyst by an Endoscopic Approach
-
Analýza dat v neurologii
LXI. Závěrečné příklady k analýzám trendů v kontingenčních tabulkách -
Zpráva o činnosti Specializační oborové rady
Neurologie v roce 2016 - Zemřel prof. MUDr. Stanislav Němeček, DrSc.
- Životné jubileum prof. MUDr. Pavla Traubnera, PhD.
- Prof. MUDr. Evžen Růžička, DrSc., FCMA, FEAN slaví 60 let
- Zpráva z výročního kongresu České neurochirurgické společnosti
- Zpráva o 9. mikrovaskulárním workshopu
- Je třeba léčit motorické tiky
- Není třeba léčit motorické tiky
-
Komentář ke kontroverzím
Je třeba léčit motorické tiky? - Stem Cell Therapy for Amyotrophic Lateral Sclerosis – an Overview of Current Clinical Experience
- Editorial
- Genetics of Atypical Parkinsonism
- Current Perception of Contraindications and Complications of Nerve Conduction Studies and Needle Electromyography
- The Evaluation of Corneal Innervation Using Corneal Confocal Microscopy
- Diabetic Retinopathy and Changes in Corneal Nerve Fibers Assessed by Confocal Microscopy
- Validation of Myasthenia Gravis Quality of Life Questionnaire – Czech Version of MG-QOL15
- Periodic Limb Movements During Sleep are More Severe in Narcolepsy with Cataplexy than in Narcolepsy without Cataplexy
- An Association Between Early Metabolic Changes in the Brain and Selected Baseline Parametres in Patients after Subarachnoid Haemorrhage Due to Ruptured Intracranial Aneurysm
- Using Transcranial Sonography to Display Intracranial Structures in the B-mode
- The Changing Microbiological Pattern in Patients with Confirmed Bacterial Meningitis after Post-craniotomy Surgery
- Essential Neurological Examination – Time for Change?
- Základní neurologické vyšetření – nastal čas pro změny?
- Základní neurologické vyšetření – nastal čas pro změny?
- Neurological and MRI Screening Improves Long-term anti-TNF-α Treatment Safety in Patients with Crohn΄s Disease
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle
- Není třeba léčit motorické tiky
- Current Perception of Contraindications and Complications of Nerve Conduction Studies and Needle Electromyography
- Essential Neurological Examination – Time for Change?
- Extirpation of Colloid Cyst by an Endoscopic Approach
