Does choroidal thickness change in Parkinson’s disease?
Mění se při Parkinsonově nemoci tloušťka cévnatky?
Cíl: Stanovit tloušťku cévnatky u pacientů s Parkinsonovou nemocí (PN) v porovnání se zdravými jedinci a vyhodnotit vztah mezi trváním onemocnění a tloušťkou cévnatky.
Materiály a metody: Studovaná skupina zahrnula 24 pravých očí 24 pacientů s PN a kontrolní skupina zahrnula 25 pravých očí 25 zdravých jedinců. Tloušťka cévnatky byla v obou skupinách měřena optickou koherentní tomografií ve spektrální doméně (SD-OCT) v 6 bodech: ve středu fovea centralis a 500µm intervalech temporálně (3 body) a nazálně (2 body) od středu fovea centralis. Skupiny byly porovnány podle hodnot tloušťky cévnatky a byly provedeny skupinové analýzy. Dále byla hodnocena korelace mezi trváním PN a tloušťkou cévnatky.
Výsledky: Střední věk 24 pacientů s PN (14 mužů) byl 67,5 ± 12,8 let a průměrný věk 25 zdravých jedinců (13 mužů) byl 66,1 ± 9,7 let. Mezi skupinami nebyly s ohledem na věk nebo pohlaví žádné významné rozdíly (p = 0,59 a p = 0,768). U pacientů s PN byly pozorovány významně vyšší hodnoty tloušťky cévnatky subfoveálně (p = 0,04), 500 µm nazálně (p = 0,03) a 500, 1 000 a 1 500 µm temporálně od středu fovea centralis (p = 0,02, p = 0,02, resp. p = 0,02, ). Mezi trváním PN a tloušťkou cévnatky nebyla shledána žádná korelace (p > 0,05).
Závěr: Tloušťka cévnatky může být u pacientů s PN vyšší v porovnání se zdravými jedinci. Pro potvrzení našich výsledků a zhodnocení souvisejících patofyziologických mechanismů jsou však zapotřebí další studie.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Klíčová slova:
tloušťka cévnatky – optická koherentní tomografie
Authors:
Ç. Öktem 1; E. Ö. Öktem 2; A. Kurt 1; R. Kilic 3
Authors‘ workplace:
Department of Ophthalmology, Alaaddin Keykubat University Alanya Education and Research Hospital, Antalya, Turkey
1; Department of Neurology, Alaaddin Keykubat University Alanya Education and Research Hospital, Antalya, Turkey
2; Department of Ophthalmology, Ahi Evran University Education and Research Hospital Kirsehir, Turkey
3
Published in:
Cesk Slov Neurol N 2019; 82(6): 677-681
Category:
Original Paper
doi:
https://doi.org/10.14735/amcsnn2019677
Overview
Aim: To evaluate the choroidal thickness (CT) in patients with Parkinson’s disease (PD) compared to healthy individuals and to investigate the relationship between the disease duration and the CT.
Materials and methods: The study group included 24 right eyes of 24 PD patients and the control group included 25 right eyes of 25 healthy individuals. The CT was measured in both groups using spectral domain optical coherence tomography (SD-OCT) at 6 points: the foveal centre and at 500-µm intervals temporal (3 points) and nasal (2 points) to the foveal centre. The groups were compared in terms of CT values and group analyses were performed. The correlation between the PD duration and the CT was also evaluated.
Results: The mean age of the 24 PD patients (14 males) was 67.5 ± 12.8 years and the mean age of the 25 healthy individuals in the control group (13 males) was 66.1 ± 9.7 years. There were no significant differences between the groups in terms of age or gender (P = 0.59 and P = 0.768). The PD patients had significantly increased CT values subfoveal (P = 0.04), 500 µm nasal (P = 0.03), and 500, 1,000 and 1,500 µm temporal to the foveal centre (P = 0.02, P = 0.02, P = 0.02, resp.). No correlation was detected between the PD duration and the CT (P > 0.05).
Conclusion: The choroidal thickness may increase in PD compared with healthy individuals. However, more studies are needed to corroborate our findings and to assess the underlying pathophysiology.
帕金森氏病的脉络膜厚度会改变吗?
目的:评估帕金森病(PD)与健康个体相比的脉络膜厚度(CT),并研究疾病持续时间与CT之间的关系。
材料和方法:研究组包括24名PD患者的24个右眼,对照组包括25名健康个体的25个右眼。两组均使用频谱域光学相干断层扫描(SD-OCT)在6个点处测量CT:中央凹中心和距中央凹中心的时间间隔(3个点)和鼻腔(2个点)间隔500 µm。比较各组的CT值,并进行组间分析。还评估了PD持续时间和CT之间的相关性。
结果:24名PD患者(14名男性)的平均年龄为67.5±12.8岁,对照组中25名健康个体的平均年龄(13名男性)为66.1±9.7岁。两组之间在年龄或性别方面无显著差异(P = 0.59和P = 0.768)。 PD患者的中央凹下CT值(P = 0.04),鼻中央500 µm(P = 0.03)以及距中央凹中心的颞侧分别为500、1,000和1,500 µm(分别为P = 0.02,P = 0.02,P = 0.02)。PD持续时间和CT之间未发现相关性(P> 0.05)。
结论:与健康人相比,PD的脉络膜厚度可能增加。然而,需要更多的研究来证实我们的发现和评估潜在的病理生理学。
关键词:脉络膜厚度–光学相干断层扫描
Keywords:
optical coherence tomography – choroidal thickness
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s and is characterized by the progressive loss of dopaminergic neurons [1,2]. It is reported to have a prevalence of 3% and an incidence of 8 – 18 per 100,000 [2]. Although the motor symptoms are more prominent, visual symptoms may be observed even in the preclinical stage [1]. Some retinal structural changes in PD have been detected with optic coherence tomography (OCT) measurements, and retinal nerve fibre layer (RNFL) and macular thinning have been reported [3 – 6]. In addition to these structural changes, functional losses such as decreased visual acuity, reduced colour vision and contrast sensitivity, and impaired electrophysiological tests have also been reported [7,8]. Therefore, it is believed that the evaluation of ocular structures such as the retina and choroid may provide important information about the severity and duration of PD. Although there are many studies on the visual symptoms of PD and the changes it causes in the retina, there are few studies in the literature on its effect on the choroid [1,9 – 11].
The choroid is located between the retina and sclera and is one of the most vascularized tissues in the human body. The choroid plays important roles in the oxygenation and feeding of the outer retina, thermal regulation of the retina, elimination of retinal waste material and the secretion of growth factors. Thus, structurally and functionally healthy choroid tissue is critical for retinal functions [12]. The recently developed enhanced depth imaging-OCT (EDI-OCT) method provides in-depth images of the choroid in addition to all retinal layers [13].
The aim of the present study was to use EDI-OCT to evaluate the CT in PD patients compared to healthy individuals and investigate whether CT is associated with PD duration.
Materials and methods
This prospective study was conducted at the Kirşehir Ahi Evran University Training and Research Hospital‘s Ophthalmology and Neurology departments between January and March 2016. The diagnosis of PD was made by neurologists the Neurology Department according to United Kingdom Parkinson’s Disease Society Brain Bank Criteria [14]. The study included 24 right eyes of 24 PD patients who were referred by neurologists and 25 right eyes of 25 healthy age - and gender-matched individuals. All patients were informed about the study and they provided written consent. The study adhered to the principles of the Declaration of Helsinki and was approved by the institutional ethics committee. All participants underwent detailed ophthalmologic examination at the ophthalmology outpatient clinic including best corrected visual acuity measurement using a Snellen chart, intraocular pressure (IOP) measurement using Goldmann applanation tonometry and slit-lamp and dilated fundus examination. The CT measurements were obtained using the EDI mode of a spectral domain OCT device (Heidelberg Engineering, Heidelberg, Germany). All measurements were performed in the morning to avoid diurnal fluctuations in CT [15]. Individuals with visual acuity of 20/ 25 or better were included in the study.
Inclusion criteria
Subjects who did not meet the exclusion criteria and had a corrected distance visual acuity of 20/ 25 or above, spherical refractive error of – 1.5 to +1.5 dioptres and an IOP of 21 mm Hg or less were included in the study.
Exclusion criteria
Patients with a refractive error > ± 1.5 dioptres, choroidal neovascularization or any other macular/ retinal diseases that might affect the vision, intraocular inflammation and/ or infection; a history of any type of intraocular surgery, trauma, serious eye disease (corneal disease, glaucoma, serious cataract) or any systematic disease that might affect the eye (such as diabetes mellitus, arterial hypertension, vasculitis); or a history of smoking and alcohol use, coffee addiction, or use of vasoactive drugs were excluded.
Optic coherence tomography protocol
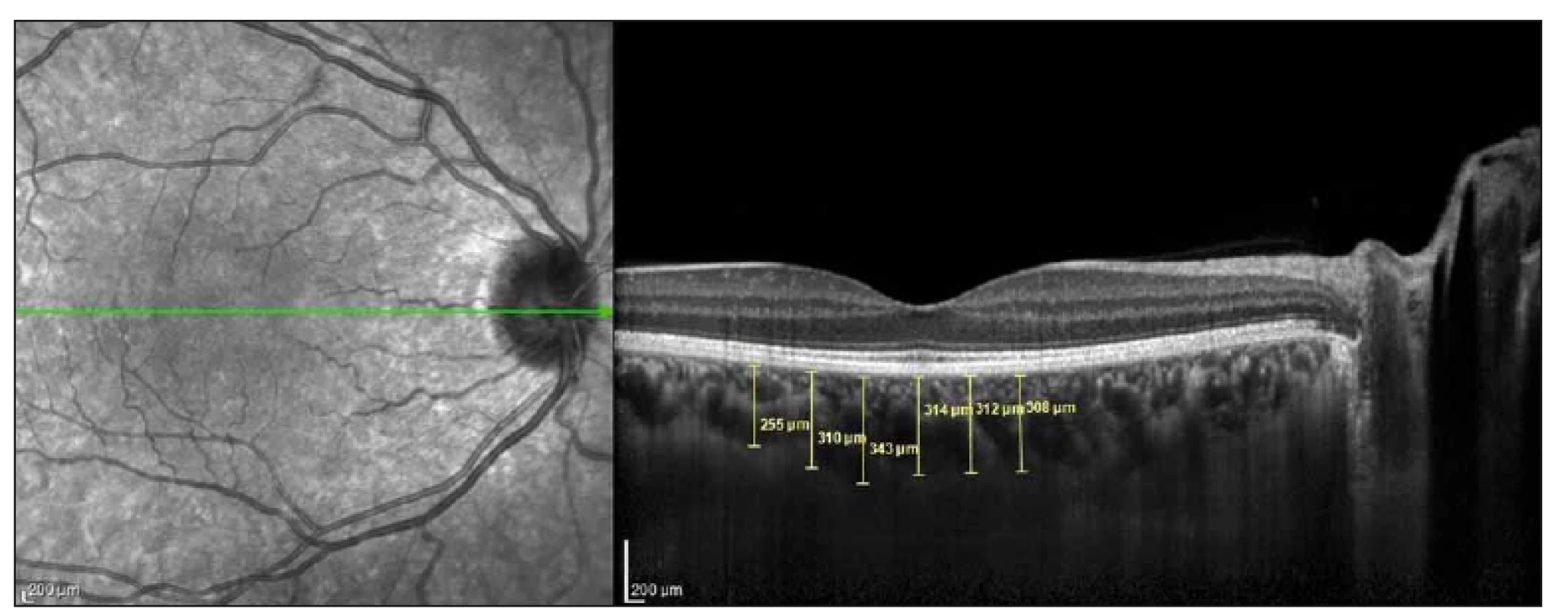
Images were acquired at λ = 840 nm, 40,000 A-scan/ s, 7 µm axial and 14 µm transverse resolution using a Heidelberg spectral domain OCT device with 6.3.3.0 software. The CT values of all participants were measured manually by the same researcher (Ç.Ö.) as the distance between the outer reflection of the retinal pigment epithelium and the inner border of the sclera using the digital marker provided by the device software in EDI-OCT mode (Fig. 1). The cross-sections were measured in the subfoveal (SF) area and at 500-µm intervals temporal and nasal of the fovea. Three measurements were obtained at 500 µm (T500), 1,000 µm (T1000), and 1,500 µm (T1500) temporal of the foveal centre. Two measurements were performed at 500 µm (N500) and 1,000 µm (N1000) nasal of the foveal centre (Fig. 1).
Obr. 1. Měření tloušťky cévnatky bylo provedeno v 6 bodech s 500μm intervaly na řezu snímku získaném optickou koherenční
tomografií.
Statistical analysis
Categorical variables were expressed as frequency and percentage values. Continuous variables were presented as mean, standard deviation, median, minimum and maximum values. The Shapiro-Wilk test was used to determine whether the continuous variables were distributed normally. The correlations between categorical variables were evaluated using Pearson’s chi-square analysis. For independent variables, intergroup comparisons were performed using the Mann-Whitney U test. Spearman correlation analysis was used to detect correlations between the variables. A P value < 0.05 was considered statistically significant. Analyses were done using NCSS 11 (Number Cruncher Statistical System, 2017 Statistical Software) and MedCalc Statistical Software version 18 (MedCalc Software bvba, Ostend, Belgium).
Results
The study group included 24 right eyes of 24 PD patients and the control group 25 right eyes of 25 subjects. The mean age of the PD patients was 67.5 ± 12.8 years, and the mean age of the control group was 66.1 ± 9.7 years. There was no significant difference between the mean age of the groups (P = 0.59). There were 10 women and 14 men in the PD group and 12 women and 13 men in the control group (P = 0.768). The mean disease duration was 3.5 ± 1.9 years in the study group (Tab. 1).
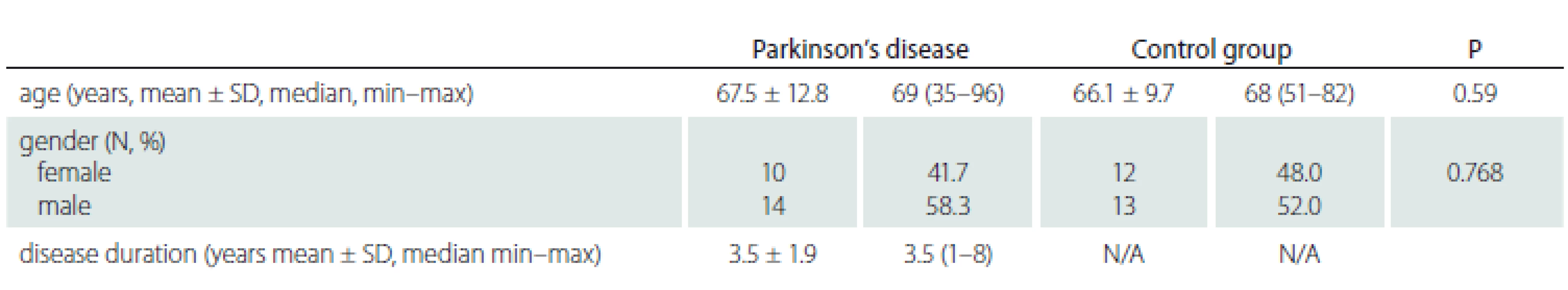
N – number; N/A – not applicable; SD – standard deviation
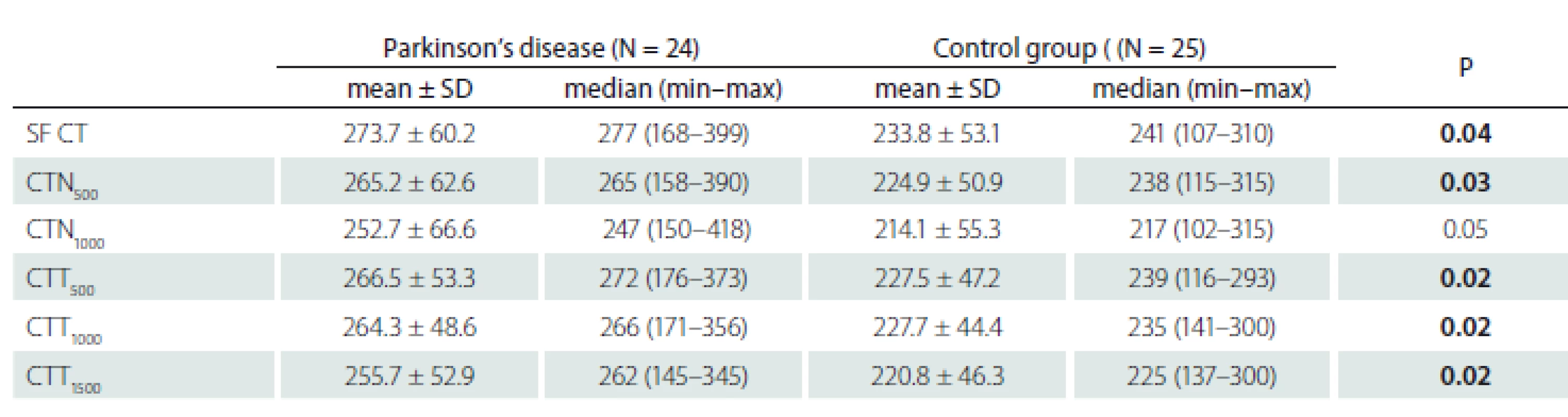
The choroid was significantly thicker in the PD group at SF (P = 0.04), N500 (P = 0.03), T500 (P = 0.02), T1000 (P = 0.02), and T1500 (P = 0.02). The difference at N1000 was not statistically significant (P = 0.05) (Tab. 2).
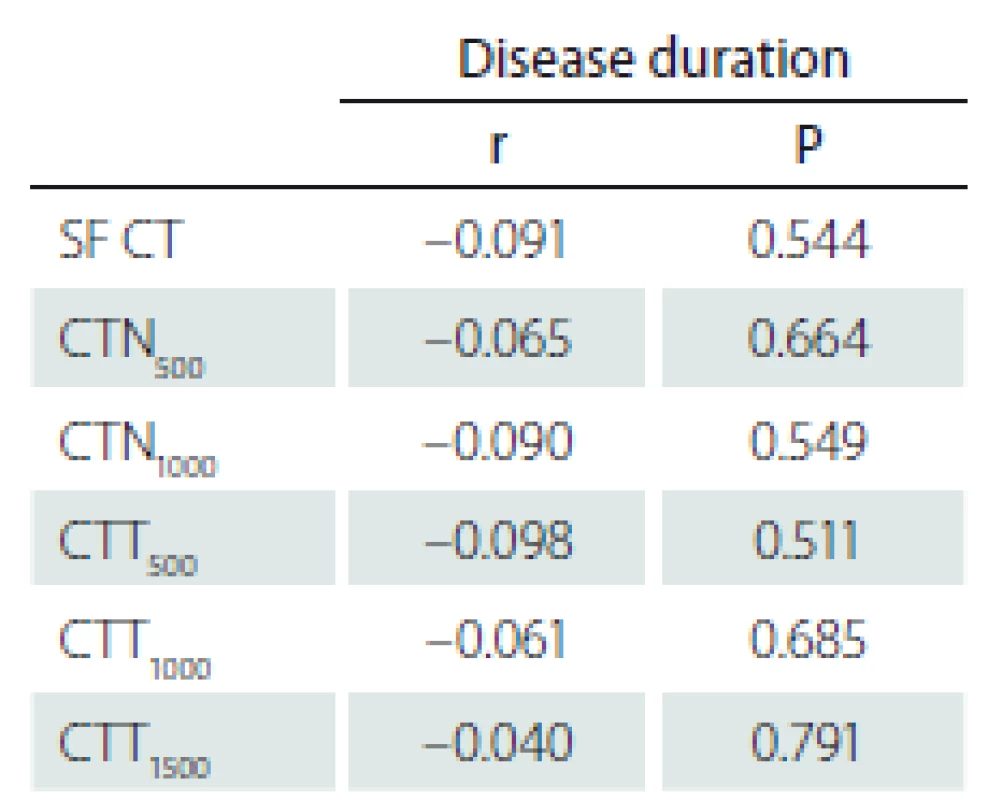
The correlation between the CT measurement and disease duration in the PD group was also investigated and no statistically significant correlation was found (SF [P = 0.544], N500 [P = 0.664], N1000 [P = 0.549], T500 [P = 0.511], T1000 [P = 0.685], T1500 [P = 0.791]) (Tab. 3).
Discussion
Visual symptoms are among the non-motor signs of PD and can be observed even in the preclinical stage of the disease [1]. Earlier studies demonstrated RNFL and macular thinning in PD patients [1,3 – 6]. Moreover, visual evoked potential and electroretinography measurements indicated foveal retinal ganglion cell damage [16]. In light of these data, the present study was designed to provide important information about the severity and duration of PD, a neurodegenerative disease, by evaluating ocular structures such as the retina and choroid. Although there are many studies on the visual symptoms of PD and the changes it causes in the retina, little research was published about its effect on the choroid [1,9 – 11]. These studies yielded conflicting results regarding structural measurements of the choroid [1,9 – 11].
In our study, the SD-OCT measurements of the CT were greater in PD patients than in the healthy subjects in all 6 points assessed. The differences were statistically significant at all points except 1,000 µm nasal of the fovea.
Eraslan et al measured the CT in 22 PD patients and 25 healthy participants using SD-OCT and determined that the choroid was thinner at all measured points in PD patients [9]. Moschos et al also used the SD-OCT to evaluate the choroid, RNFL and ganglion cell complex (GCC) thicknesses in 31 PD patients and 25 healthy individuals and reported RNFL, GCC, and choroidal thinning in all regions in PD [1].
In contrast to those two studies, a study including 50 PD patients and 54 healthy individuals showed that PD patients had higher macular and peripapillary CT values measured using swept-source OCT (SS-OCT) [11]. In another study utilizing SS-OCT, the results of 40 PD patients and 80 healthy participants were published and PD patients had greater CT values at all 4 points measured in the peripapillary region [10]. The increase in CT observed in our study is consistent with the results of these SS-OCT studies.
The researchers who observed increased or decreased CT attributed their findings to various systemic and histopathological changes observed in PD patients. PD is known to cause changes in the peripheral and autonomic nervous systems, resulting in symptomatic orthostatic hypotension in 20 – 58% of patients [17]. Moreover, dopamine agonists used in the treatment of PD were reported to cause arterial hypotension [18]. Eraslan et al stated that lower CT in PD could be due to irregularities in the choroidal blood flow associated with hypoperfusion and the hypotensive effect of dopamine agonists used in PD treatment [9]. Moschos et al reported that thinning of the choroidal tissue could occur as a result of the synergistic effect of vascular anomalies and neurodegeneration [1].
However, a clinicopathological study found hyaline thickening in the white matter of the brain and enlarged perivascular spaces in PD patients compared with healthy individuals [19]. The results of this study support an increased CT in PD patients. Consistent with the literature, Satue et al observed an increased CT in PD patients and stated that although ultrastructural changes in the choroidal tissue in PD could not be fully explained, the increase in the CT despite hypoperfusion in these patients might be due to changes in the density of perivascular connective tissue [11].
In our study, we found that the choroid was significantly thicker in PD patients at most of the measured points. This may be attributed to the perivascular enlargement and increase in connective tissue reported in PD patients in the above-mentioned studies. This hypothesis can only be definitively tested by histopathological examination of the choroid. The choroid is known to be vitally important for healthy retinal tissue. It plays a key role in oxygenating and feeding the outer retina, eliminating retinal waste and secreting growth factors [12]. Therefore, thickening of the vascular choroidal tissue that supports the retina may occur to compensate for the retinal atrophy observed in PD patients.
In the present study, we also investigated whether CT was associated with PD duration. To the best of our knowledge, the correlation between disease duration and CT was previously evaluated only by Eraslan et al, who reported that choroidal thinning was associated with disease duration [9]. Unlike this article, there was no significant correlation between the PD duration and CT in our study.
Our study has some limitations. First, we questioned our patients regarding the presence of arterial hypertension and used it as an exclusion criterion. However, we did not ask about arterial hypotension. Hypotension and hypoperfusion due to autonomic dysfunction are common in PD [18]. This could also affect the choroidal perfusion. The second limitation is that we did not determine the stage of PD. Therefore, our study group may not have been homogeneous. Furthermore, we did not measure axial length in our patients but excluded patients with myopia and hypermetropia with a spherical equivalent of ± 1.5 or higher to minimize the effect of axial length. Another possible limitation is that the sample size of our study was relatively small.
In conclusion, CT may increase in PD due to ultrastructural changes such as perivascular enlargement and choroidal compensation for atrophic changes in the retina. However, more studies with larger patient groups and fewer limitations are needed to corroborate our findings.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Çağlar Öktem, MD
Department of Ophthalmology
Alaaddin Keykubat University
Alanya Education and Research Hospital
074 00 Antalya
Turkey
e-mail: cglroktm@gmail.com
Accepted for review: 12. 8. 2019
Accepted for print: 30. 10. 2019
Sources
1. Moschos MM, Chatziralli IP. Evaluation of choroidal and retinal thickness changes in Parkinson‘s disease using spectral domain optical coherence tomography. Semin Ophthalmol 2018; 33(4): 494 – 497. doi: 10.1080/ 08820538.2017.1307423.
2. Archibald NK, Clarke MP, Mosimann UP et al. The retina in Parkinson‘s disease. Brain 2009; 132(Pt 5): 1128 – 1145. doi: 10.1093/ brain/ awp068.
3. Aaker GD, Myung JS, Ehrlich JR et al. Detection of retinal changes in Parkinson’s disease with spectral-domain optical coherence tomography. Clin Ophthalmol 2010; 4 : 1427 – 1432. doi: 10.2147/ OPTH.S15136.
4. Archibald NK, Clarke MP, Mosimann UP et al. Retinal thickness in Parkinson’s disease. Parkinsonism Relat Disord 2011; 17(6): 431 – 436. doi: 10.1016/ j.parkreldis.2011.03.004.
5. Moschos M, Tagaris G, Markopoulos I et al. Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur J Ophthalmol 2011; 21(1): 24 – 29. doi: 10.5301/ EJO.2010.1318.
6. Inzelberg R, Ramirez JA, Nisipeanu P et al. Retinal nerve fiber layer thinning in Parkinson disease. Vision Res 2004; 44(24): 2793 – 2797. doi: 10.1016/ j.visres.2004.06.009.
7. Weil RS, Schrag AE, Warren JD et al. Visual dysfunction in Parkinson‘s disease. Brain 2016; 139(11): 2827 – 2843. doi: 10.1093/ brain/ aww175.
8. London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol 2013; 9(1): 44 – 53. doi: 10.1038/ nrneurol.2012.227.
9. Eraslan M, Cerman E, Yildiz Balci S et al. The choroid and lamina cribrosa is affected in patients with Parkinson‘s disease: enhanced depth imaging optical coherence tomography study. Acta Ophthalmol 2016; 94(1): e68 – e75. doi: 10.1111/ aos.12809.
10. Garcia-Martin E, Pablo LE, Bambo MP et al. Comparison of peripapillary choroidal thickness between healthy subjects and patients with Parkinson‘s disease. PLoS One 2017; 12(5): e0177163. doi: 10.1371/ journal.pone.0177163.
11. Satue M, Obis J, Alarcia R et al. Retinal and choroidal changes in patients with Parkinson‘s disease detected by swept-source optical coherence tomography. Curr Eye Res 2018; 43(1): 109 – 115. doi: 10.1080/ 02713683. 2017.1370116.
12. Zengin MÖ, Karahan E, Özyurtlu F et al. The effect of blood pressure regulation on choroidal thickness. Ret Vit 2014; 22(3): 213 – 216.
13. Erol MK, Coban DT, Ceran BB et al. Enhanced depth imaging optical coherence tomography and fundus autofluorescence findings in bilateral choroidal osteoma: a case report Arq Bras Oftalmol 2013; 76(3): 189 – 191. doi: 10.1590/ S0004-27492013000300012.
14. Reichman H. Clinical criteria for the diagnosis of Parkinson’s disease. Neurodegener Dis 2010; 7(5): 284 – 290. doi: 10.1159/ 000314478.
15. Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci 2011; 52(8): 5121 – 5129. doi: 10.1167/ iovs.11-7364.
16. Bodis-Wollner I. Retinopathy in Parkinson disease. J Neural Transm (Vienna) 2009; 116(11): 1493 – 1501. doi: 10.1007/ s00702-009-0292-z.
17. Velseboer DC, de Haan RJ, Wieling W et al. Prevalence of orthostatic hypotension in Parkinson‘s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 2011; 17(10): 724 – 729. doi: 10.1016/ j.parkreldis.2011.04.016.
18. Senard JM, Brefel-Courbon C, Rascol O et al. Orthostatic hypotension in patients with Parkinson‘s disease: pathophysiology and management. Drugs Aging 2001; 18(7): 495 – 505. doi: 10.2165/ 00002512-200118070-00003.
19. Schwartz RS, Halliday GM, Cordato DJ et al. Small-vessel disease in patients with Parkinson‘s disease: a clinicopathological study. Mov Disord 2012; 27(12): 1506 – 1512. doi: 10.1002/ mds.25112.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2019 Issue 6
-
All articles in this issue
- Gunshot injury of the brain
- Are ticagrelor and prasugrel an alternative in the antiplatelet treatment of ischemic stroke? YES
- Are ticagrelor and prasugrel an alternative in the antiplatelet treatment of ischemic stroke? NO
- Are ticagrelor and prasugrel an alternative in the antiplatelet treatment of ischemic stroke?
- Cervical plexus lesions in clinical practice
- Neurorehabilitation of gait impairment using functional electrical stimulation – current findings from randomized clinical trials
- Difficulty in respecting autonomy in patients with Parkinson’s disease
- Current management of patients with degenerative cervical spine compression
- Surgical treatment of bilateral drug-resistant Menière’s disease
- Mechanical thrombectomy in the treatment of acute ischemic stroke in childhood
- Doporučení pro mechanickou trombektomii akutního mozkového infarktu – verze 2019
- Osmdesátiny doc. MU Dr. Jiřího Bauera, CSc.
- Intraplaque hemorrhage in symptomatic and asymptomatic progressive internal carotid artery stenosis – a pilot study
- Significant fall risk factors in the personal history of in-patients with neurological disease
- Spinal meningiomas – 92 patients operated at our department
- Civilian and military gunshot wounds to the head
- Epidural application of steroids Part 1 – Patient profile before application
- Facial nerve reconstruction with great auricular nerve graft following resection of recurrent basal cell carcinoma in parotidomasseteric region
- A comparison of perioperative pressure measurements in the aneurysm sac and parent artery in ruptured and unruptured aneurysm
- A systematic review of the clinical efficacy of sacroiliac joint stabilization in the treatment of lower back pain
- Does choroidal thickness change in Parkinson’s disease?
- Conservative management of a ruptured Galassi III middle fossa arachnoid cyst
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Cervical plexus lesions in clinical practice
- Doporučení pro mechanickou trombektomii akutního mozkového infarktu – verze 2019
- Mechanical thrombectomy in the treatment of acute ischemic stroke in childhood
- Gunshot injury of the brain
