Modern microsurgery as a permanent, safe and gentle solution of unruptured intracranial aneurysms
Authors:
J. Šroubek 1; J. Hanuška 1; O. Šoula 1; R. Tomáš 1; R. Bruna 2; J. Klener 1
Authors‘ workplace:
Neurochirurgické oddělení, Nemocnice Na Homolce, Praha
1; Klinika zobrazovacích metod 2. LF UK a FN Motol, Praha
2
Published in:
Cesk Slov Neurol N 2019; 82(2): 176-182
Category:
Original Paper
doi:
https://doi.org/10.14735/amcsnn2019176
Overview
Aim: Unruptured intracranial aneurysms (UIA) are getting more frequently diagnosed in neurology and neurosurgery clinics as the quality and availability of diagnostic radiology methods increases. Due to the risk of rupture and bleeding, aneurysm occlusion should be considered. The indication and the way of treatment are not yet fully agreed upon. We present results for UIA surgeries that were done during the last 9 years at our department and our approach on this topic.
Patients and methods: Patients with UIA that were operated on at our department were retrospectively analyzed with special consideration of procedure safety and full obliteration of the aneurysm.
Results: 146 patients with 184 incidental or coincidental aneurysms were operated on during the last 9 years. The most common localization was on the middle cerebral artery (40%). Perioperative complication occurred in 10% of cases and total surgery morbidity/ mortality was 3.5/ 0%. CTA examination performed in 143 patients proved full obliteration of the aneurysm in 99% cases. The average follow-up of the patients was 4.5 years.
Conclusion: Our results demonstrated that microsurgical treatment of UIAs was safe, effective in the long term and tolerated well by patients. Preferentially in UIA cases, where the main goal is to eliminate the risk of bleeding, surgery should always be considered as a therapeutic modality.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
现代显微外科是一种永久性、安全、温和的治疗未破裂颅内动脉瘤的方法
目的:
随着诊断放射学方法的质量和可用性的提高,未破裂颅内动脉瘤(UIA)在神经内科和神经外科诊所的诊断越来越频繁。由于动脉瘤破裂出血的风险,应考虑动脉瘤闭塞。适应症和治疗方法尚未完全一致。我们介绍了过去9年在我们部门进行的UIA手术的结果和我们在这个主题上的方法。
患者和方法:
回顾分析我科手术的UIA患者,特别考虑手术的安全性和动脉瘤的完全闭塞。
结果:
在过去的9年中,共有146名病人接受了184个动脉瘤的手术治疗。最常见的定位在大脑中动脉(40%)。围手术期并发症发生率为10%,手术总发病率/死亡率为3.5% / 0%。143例CTA检查证实动脉瘤完全闭塞,占99%。患者的平均随访时间为4.5年。
结论:
我们的研究结果表明,显微外科治疗UIAs是安全的,长期有效的,患者耐受性良好。在UIA病例中,主要目标是消除出血的风险,手术应始终被视为一种治疗方式。
关键词:
未破裂的颅内动脉瘤夹闭术
Keywords:
unruptured intracranial aneurysm – clipping
Introduction
Due to the increasing availability and quality of imaging methods, unruptured intracranial aneurysms (UIA) are more frequently diagnosed in neurological and neurosurgical practice. Two major clinical issues arise: 1. which aneurysms require intervention and 2. how to treat the aneurysm (microsurgically or endovascularly). Neither of these issues are clearly resolved in contemporary literature. Scoring systems based on meta-analyses of relevant publications have been created to estimate the likelihood of hemorrhage for a particular patient with a particular aneurysm (e.g., PHASES [1], UIATS [2]), thereby assisting the clinician to address the first issue. With regard to treatment method, the lower long-term effectiveness of endovascular methods with comparable risk has been emphasized [3]. In microsurgery, emphasis has been placed on the use of modern microsurgical methods, which are less invasive and result in lower morbidity [4].
In the present publication we outline the possibilities of modern microsurgical treatment, discuss the indications for intervention in UIAs, and present microsurgical treatment as a safe method with long-term efficacy on our cohort of patients.
Possibilities of modern microneurosurgery
Similar to the evolution of endovascular methods, the evolution of modern microsurgical methods allows even safer isolation the aneurysm from the circulation.
Newer methods include:
- reducing invasiveness by using smaller operative approaches and non-retraction techniques (Fig. 1);
- the liberal use of temporary clipping methods, pilot clip application, and remodeling of the aneurysm using bipolar coagulation;
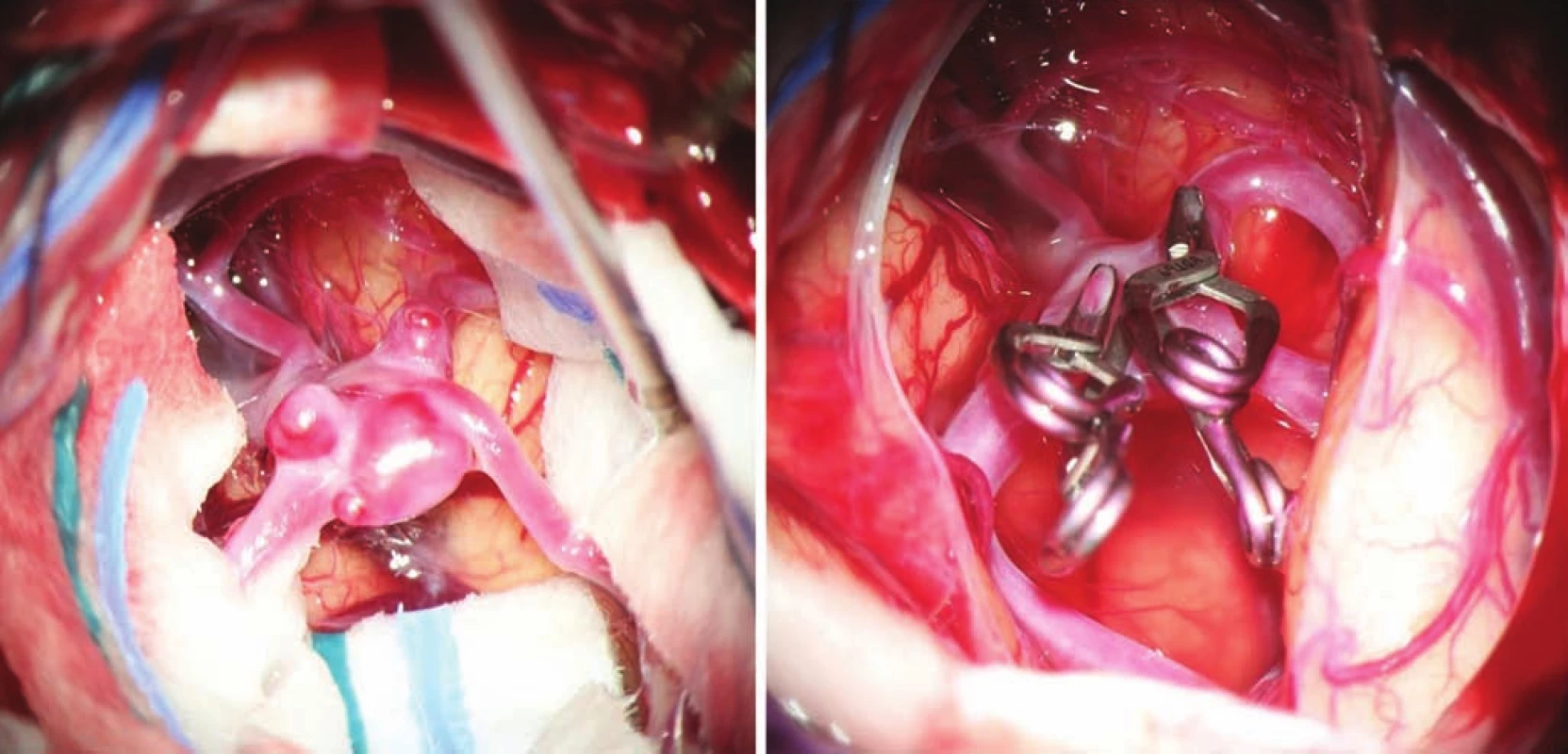
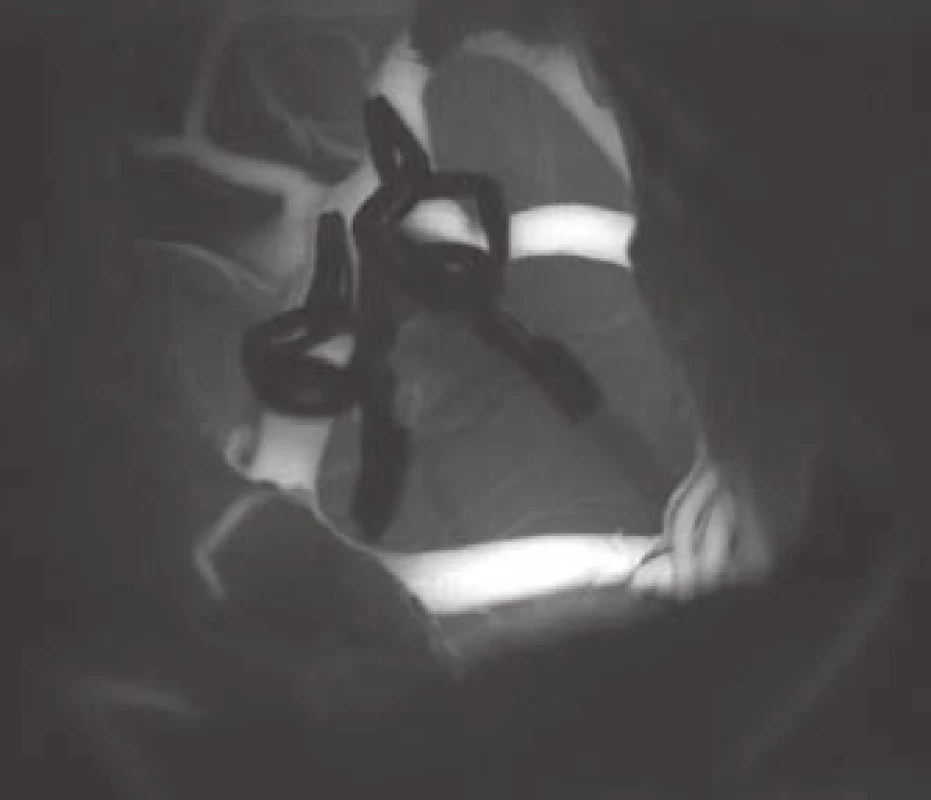
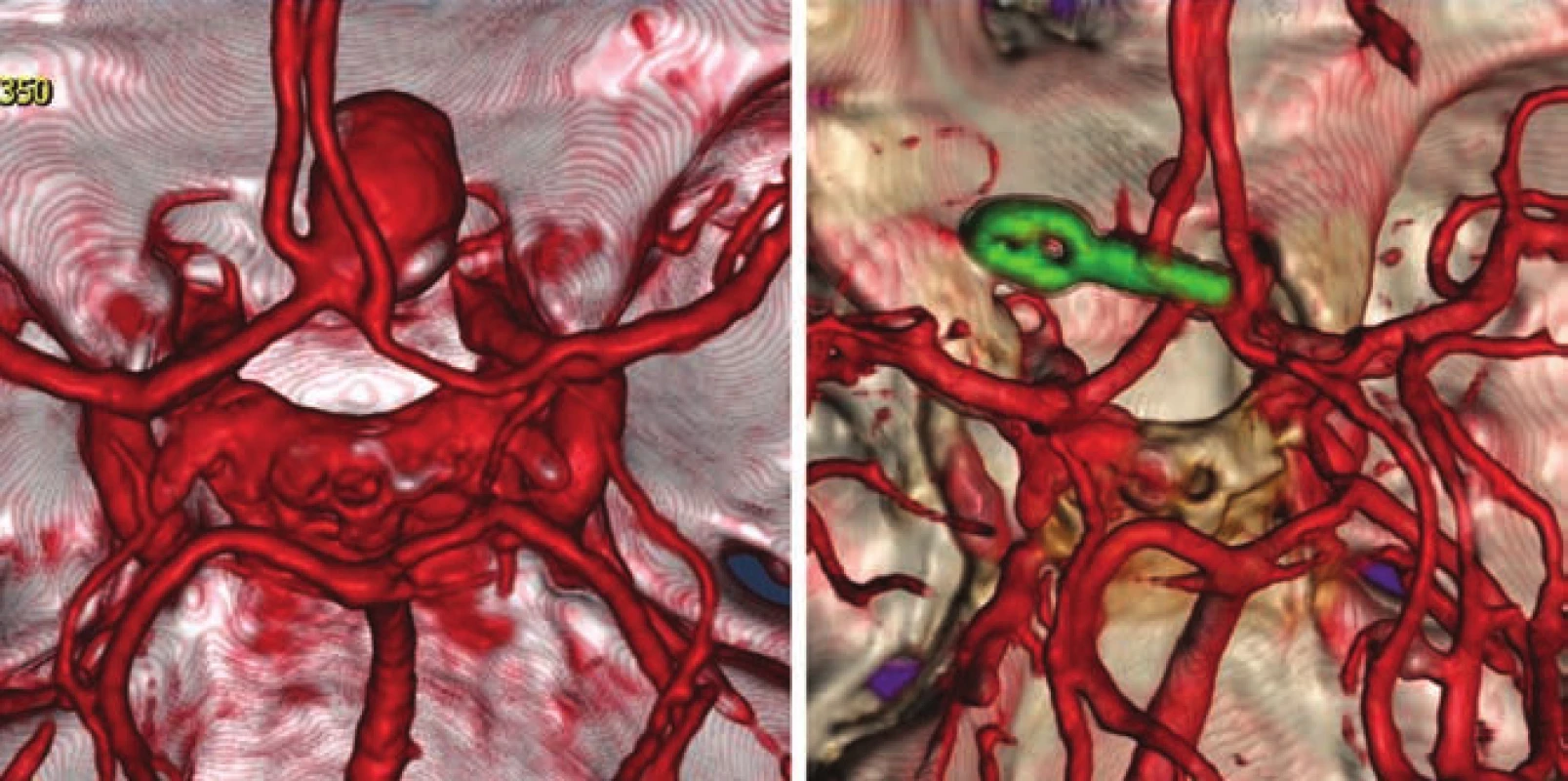
- the use of modern methods to maintain the patency of major vessels and perforators by means of intraoperative videoangiography, microdopplerography and quantitative flowmetry (Fig. 2, 3);
- the use of electrophysiological monitoring;
- the possibility of using short-term cardiac arrest by applying adenosine or rapid ventricular pacing methods;
- the use of skull base surgical techniques, such as drilling of the anterior clinoid process, orbitozygomatic approach, far lateral approach;
- the use of low and high flow bypasses;
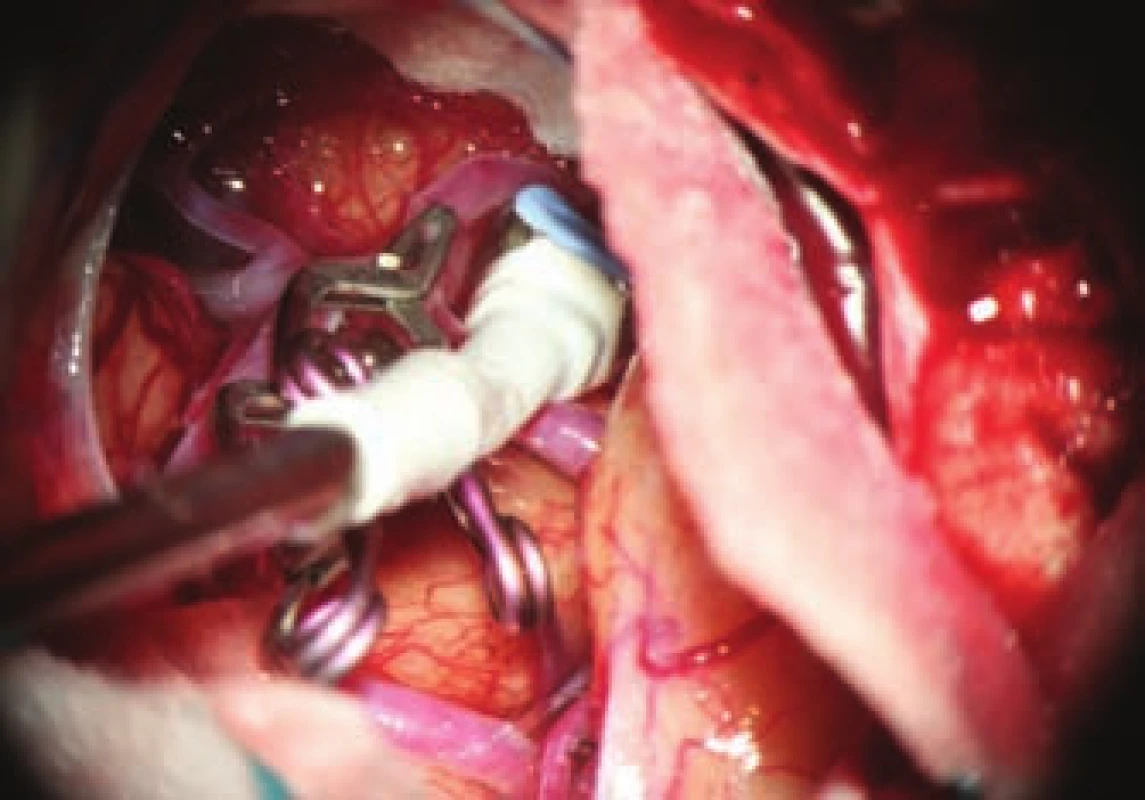
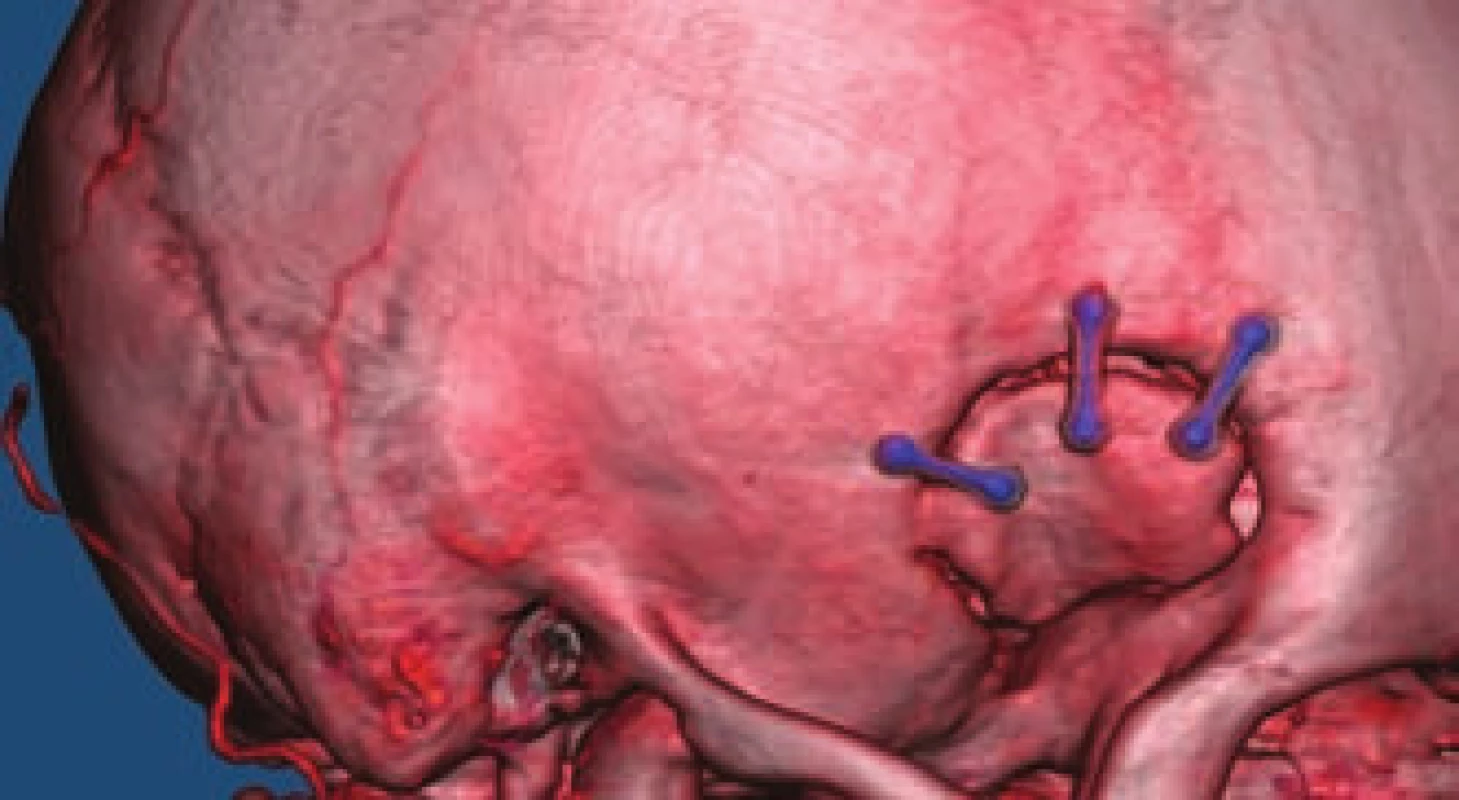
- the use of titanium clips for better and non-invasive postoperative monitoring (Fig. 4).
Non-retraction surgery requires adequate patient positioning, precise craniotomy, early evacuation of the cerebrospinal fluid (CSF) and gentle arachnoid preparation, allowing most UIAs to be operated on without the use of rigid retractors. The advantage of this physiological approach to operating using gravity and dynamic instrumentation is not only the prevention of the clearly documented risk of brain damage by rigid retractors, but especially enables the continuous sensing of brain tissue during surgery.
The position of the patient's head is very important, as the principle is to position the head in such a way that gravity pulls the brain away from the skull base and creates a spontaneous space for preparation. The adequate performance of the mini-pterional craniotomy (Fig. 5) with removal of the lesser wing of the sphenoid bone is equally important, thereby obtaining additional preparation space. After opening the dura, we generally continue by dissecting the Sylvian fissure, thereby augmenting the space previously obtained by resecting the lesser wing of the sphenoid bone. The next procedure depends on the localization of the aneurysm; for a simpler aneurysm of the bifurcation of the middle cerebral artery (MCA), a limited approximately 15 mm opening of Sylvian fissure with direct access to the aneurysm and associated vessels is sufficient. For most paraclinoid aneurysms, the drilling of the anterior clinoid is necessary followed by opening of the optic nerve sheath with its mobilization, and release of the distal dural ring of the internal carotid artery (ICA). The key to sufficient exposure of aneurysms of the anterior communicating artery (ACoA) is dissection of the interhemispheric fissure and perforation of the lamina terminalis, allowing the evacuation of CSF from the third ventricle and further relaxation of the brain.
In aneurysms of the distal anterior cerebral arteries (DACA) we use an interhemispheric approach with the application of similar principles. For aneurysms of the posterior inferior cerebellar arteries (PICA) we use the standard "far lateral" approach, for complex aneurysms of the anterior circulation rarely an orbitozygomatic approach.
The result of careful microsurgical dissection is the exposure of the neck of the aneurysm and at best preparation of the entire sac. This can give a perfect overview of the anatomical situation, in particular the relation of the neck of the aneurysm to the major arteries and perforators. By applying a temporary clip to the intake artery, we reduce the filling of the sac with blood, allowing easier and safer manipulation of the sac, remodeling by bipolar coagulation, and easier application of a pilot or permanent clip. In the case of substantial collateral circulation, temporary clips should also be applied to the efferent branches. The safe period for temporary clipping is limited to several minutes (depending on localization and status of collateral circulation); the principle is to increase the blood pressure to 120% of the initial within this period. Sac remodeling involves the use of bipolar coagulation to reduce the size and adjust the shape of larger thin-walled sacs by thermally shortening elastic fibers in the sac wall. The sac is thus remodeled to a reduced size, allowing safer application of the final clip. Applying a pilot clip involves applying a clip that does not completely close the sac but changes its shape to allow for easier and safer application of the final clip. The final clip must enclose the sac completely without residual filling or leaving the abnormal wall outside of the clip while maintaining sufficient flow in the major arteries and perforators. In many cases it is necessary to use more clips or special clips (e.g., fenestrated).
An important part of microsurgical treatment of aneurysms is the possibility to verify occlusion of the sac and in particular, verifying the patency of related vessels. Occlusion of the aneurysm is best verified by puncture of the sac. In cases where puncture with potential hemorrhage would be risky, perioperative videoagiography may be used. However, a small amount of sac filling may be present even in the absence of sac fluorescence, and thus this method is not completely reliable in this respect. It is extremely important to verify sufficient flow in the associated vessels. The basic method is perioperative videoangiography (intravenous application of fluorescent indocyanine green dye, whose intravascular filling can be monitored using a special filter on the operative microscope). Its benefits are comparable with perioperative catheter angiography with a number of advantages: non-invasive, possible to repeat the examination, and visualization of perforators. The limitation of the method is that we can only verify the patency of vessels seen directly in the microscope. Furthermore, it is not a quantitative method, and vessel narrowing, and flow reduction may potentially not be detected. This deficiency can be avoided by using flowmetry to obtain quantitative information about flow and flow direction. However, the probe is relatively bulky and, in some cases, cannot be safely applied to the vessels under investigation. Microdopplerography also provides information on blood flow through vessels. In practice, a combination of all the above-mentioned methods proves to be effective, in combination with electrophysiological monitoring in at-risk cases. Motor and somatosensory evoked potential monitoring provides information about the functional integrity of structures in relevant vascular territories.
Intravenous administration of adenosine allows for short-term (seconds to tens of seconds) reversible extreme bradycardia up to cardiac arrest with a marked decrease in pressure leading to hemostasis and collapse of the aneurysmal sac. This can help to resolve a crisis situation, for example, in perioperative aneurysm rupture, or to facilitate the preparation or application of a clip where temporary clipping is not possible. The disadvantage is the unpredictable length of "useful" arrest in a particular patient. An alternative method is rapid ventricular pacing, where a pre-established stimulation cardiac electrode induces extreme tachycardia with marked pressure drop. The advantage is the possibility of regulating the interval of hypotension, the disadvantage is the necessity of preoperative introduction of the stimulation electrode, which therefore renders this method unsuitable for "unexpected" crisis situations [5].
In complex, particularly gigantic or fusiform aneurysms, it is necessary in some cases to increase (“flow augmentation”) or replace blood flow to the endangered vessels. To accomplish this, low-flow and high-flow bypasses are used to supply blood via an alternate pathway to the endangered vessels.
Methods
The present retrospective study included all patients surgically treated at our Neurosurgical department for UIAs from January 2008 to September 2017. All surgical procedures were performed by the first and last author of this work. Coincidental aneurysms, which were treated after stabilization of the neurological status, were also included in the sample. Gigantic aneurysms were not included due to the risk of hemorrhage and the fact that their treatment differs greatly from normal saccular aneurysms.
Demographic characteristics, clinical status, mRS, (cigarette) smoking status, hypertensive status, localization, size and shape of the aneurysm (according to digital subtraction angiography (DSA) or CTA were determined in all patients. The size of the aneurysm was determined by measuring the largest diameter of the sac.
Indocyanine green (ICG) videoangiography OPMI Pentero 800 (Carl Zeiss Meditec AG, Berlin, Germany) was available for all operations and an ultrasonic flowmeter HT 331 (Transonic System Inc, Ithaca, NY, USA) was available for the last 85 patients. In the case of the flow meter, we evaluated its influence on clipping strategy. Electrophysiological monitoring was available at the discretion of the surgeon. Titanium microsurgical clips YASARGIL Aesculap Braun (Aesculap AG, Tuttlingen, Germany) were used for clipping.
After surgery, we monitored the postoperative course and complications, mortality and annual morbidity (modified Rankin Scale (mRS > 2). In addition, we noted any worsening of status related to the procedure reported by the patient or family during annual follow-up.
CTA follow-up was performed within the first year of the post-operative period (Toshiba Aquilion 64 CT scanner [Toshiba, Tokyo, Japan], work station Vitrea V6.0 – display console V4.62ER004 [Vital Images Inc., Mennetonka, MN, USA]). An additional evaluation by CTA was performed after 3–5 years.
Results
Over a period of 9 years (2008–2016), 146 patients were surgically-treated at our neurosurgery department for 184 UIAs. Most patients had no history of subarachnoid hemorrhage (130 patients). In the remaining 16 patients, UIAs were coincidentally found after treatment for other hemorrhagic aneurysms. A total of 145 patients that underwent annual follow-up were included in the analysis.
The median age of the entire group was 55 years (range 31–72 years). There was a female predilection (2.5 : 1), cigarette smokers outweighed non-smokers (3.1 : 1), and patients with hypertension were more frequently affected than normotensive patients (2.1 : 1). The overall mean follow-up period was 4.5 years.
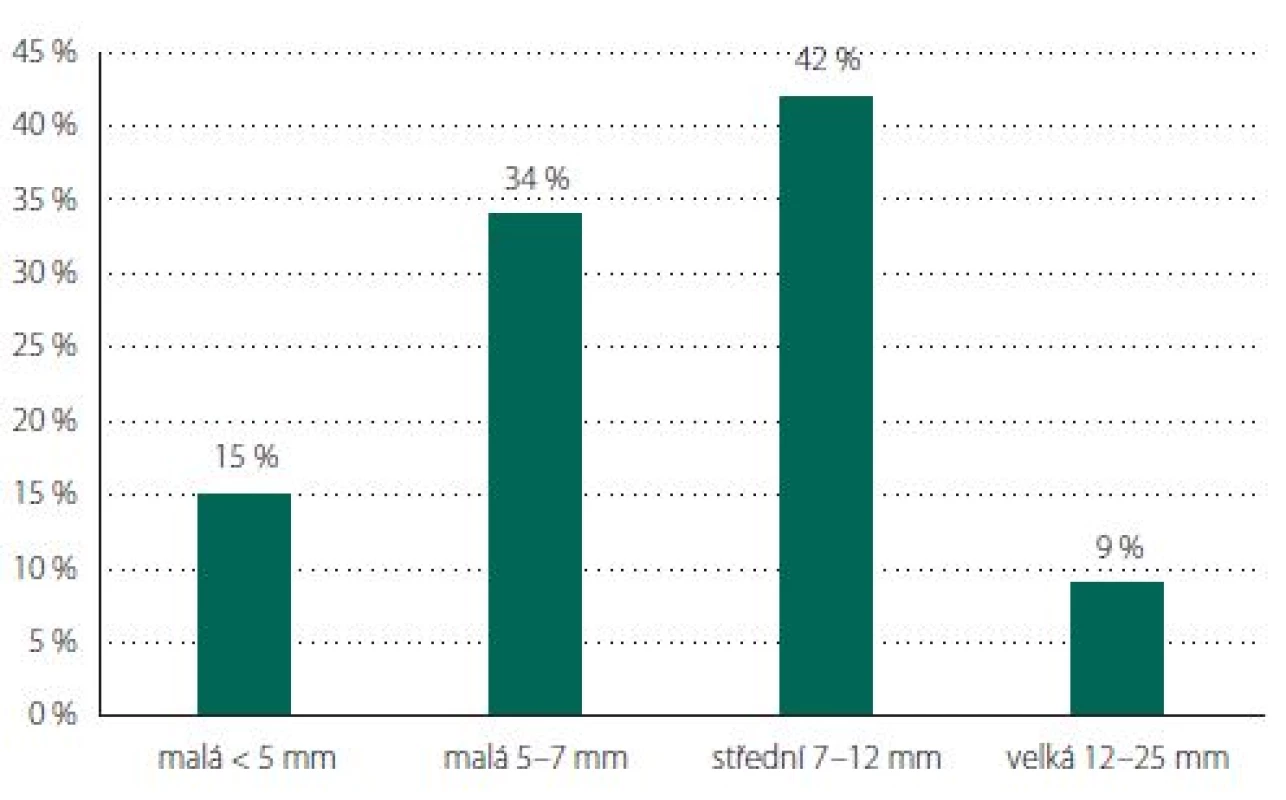
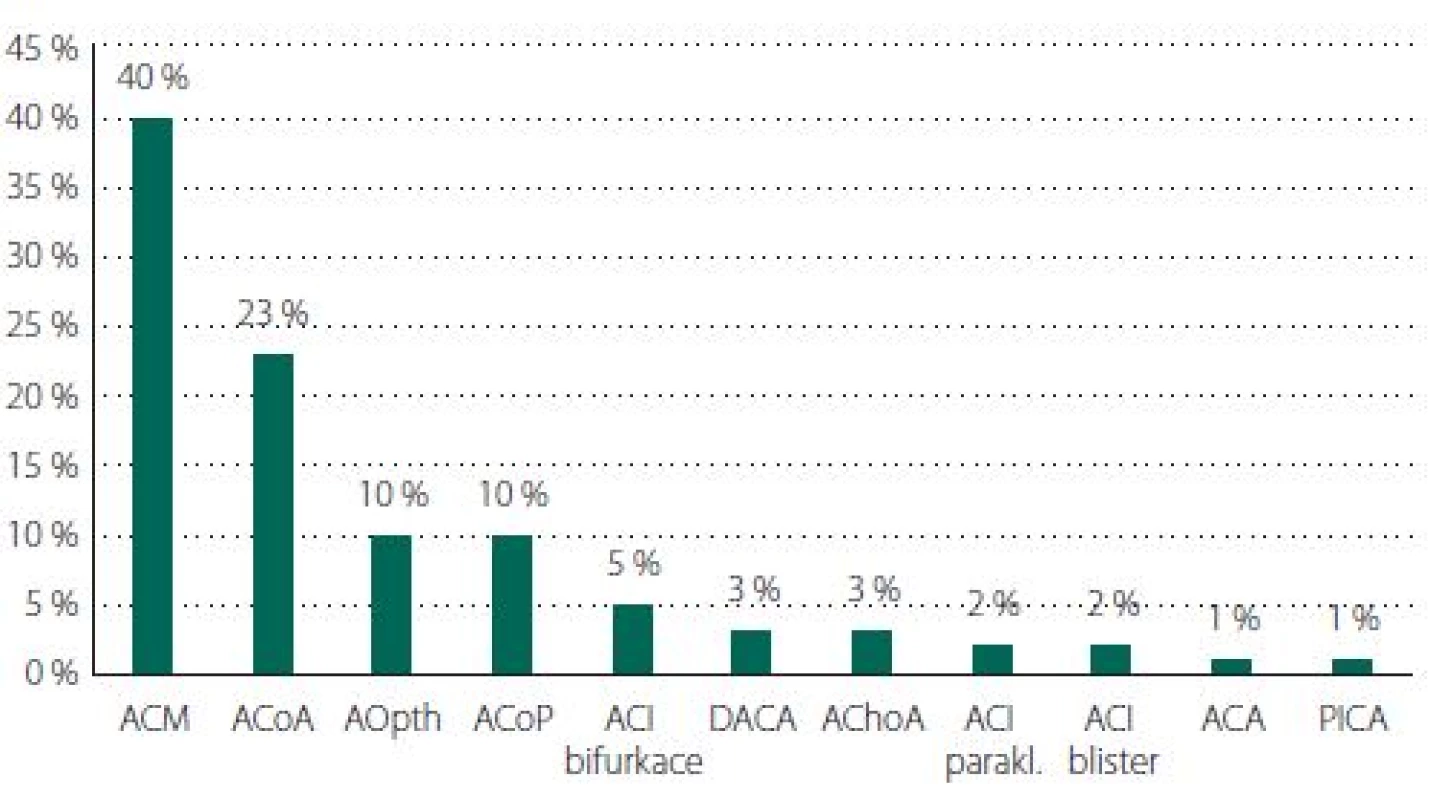
Aneurysms of the MCA predominated (N = 74, 40%), followed by ACoA (N = 42, 23%), PCoA (N = 19, 10%) and ophthalmic artery (OA; N = 19, 10%) (Tab. 1, Fig. 6). The average size for incidental aneurysms was 8 mm and for coincidental aneurysms 5 mm (Tab. 2, Fig. 7). Aneurysms under 5 mm were coincidental, multiple, or risk factors (e.g., female cigarette smokers, irregular shape) were present. Irregular shape or daughter sac occurred in 88 (49%) aneurysms.
Multiple aneurysms were treated in 15 patients from 1 craniotomy and in 10 patients from 2 craniotomies. A total of 156 craniotomies were performed for 184 UIAs. Extradural drilling of the anterior clinoid process was performed in all ophthalmic and paraclinoid aneurysms, intradural or extradural partial resection of the clinoid process was performed in 6 PCoA aneurysms.
A temporary clip was applied to the feeding artery in 45 aneurysms (of which, 4 times to the neck of the ICA in ophthalmic aneurysms). Aneurysmal sacs were remodeled 22 times.
We used ICG videoangiography to confirm the exclusion of the aneurysm in 151 cases. Residual filling was demonstrated in 8 cases with the need to move or add another clip or apply a booster clip due insufficient closure of the original clip. In 5 cases, failure of branch vessel or perforator filling was detected with ICG, followed by favorable findings after clip reposition.
Electrophysiological monitoring was used for 22 MCA aneurysms. One patient experienced a decrease in evoked potentials after applying a temporary clip to M1, requiring clip loosening and full restitution of potentials with favorable clinical findings. In contrast, a patient with a complex aneurysm at the MCA bifurcation (with low-flow bypass) developed symptomatic ischemia in region of the basal ganglia resulting in mild hemiparesis, despite normal evoked potentials during the procedure.
One hundred and forty-one (90%) operations were without postoperative complications with a favorable clinical course. Fifteen craniotomies (10%) were accompanied by postoperative complications, of which 9 (6%) had no effect on clinical status one year after the procedure (1 case pneumonia, 3 chronic subdural hematoma, 1 CSF leak, 1 oculomotor paresis, 1 worsening vision, 2 ischemia). Mortality was zero. At annual follow-up of 145 patients, 5 (3.5%) patients had accented neurological findings compared to preoperative status, of which 2 (1.5%) had severe morbidity (mRS > 2; Tab. 3). Annual follow-up examination was not performed in 1 patient with a favorable post-operative course, who died 10 months after the operation for unknown reasons (autopsy not performed due to request of family). This patient did not have follow-up CTA.
On follow-up CTA, residual sac filling was found in 1 (0.5%) patient with an aneurysm at the MCA bifurcation. Follow-up CTA was not performed in 5 patients, and in the other 139 patients complete exclusion of the aneurysm from the circulation was proven. There was no clip dislocation found in any patient. An additional CTA examination was performed after 3–5 years in 62 patients with identical findings of excluded aneurysms. The patient with incomplete aneurysm exclusion undergoes regular two-year examinations and the residual aneurysm does not increase in size.
Discussion
The prevalence of UIAs in the population is relatively high at 2–3% [6] and the number of incidentally diagnosed UIAs increases with higher numbers of MRI and CT examinations performed. Therefore, the attending physician is increasingly exposed to the issue of further action.
It is primarily necessary to assess the risk of hemorrhage in a particular UIA in each particular patient. Risk is mainly related to the size and localization of the aneurysm and is higher in the case of previous hemorrhage from other aneurysms, multiple UIAs, in the presence of daughter sacs or irregular shape, and with evidence of aneurysm growth or symptomatic manifestation. The risk of hemorrhage is also higher in smokers, hypertensive patients, women and elderly patients. Based on analysis of methodically high-quality prospective studies [7,8,9], Greving et al proposed the PHASES scoring system [1], which can be used as a guide in determining the annual risk of rupture. This risk is then compared with the risk of treating a specific aneurysm and, of course, with overall patient status and life expectancy. Relatively complicated mathematical models can also be used [10].
According to the latest recommendation of the European Stroke Organization [11], it is advisable to consider treating any aneurysm in patients under 60 years of age, and aneurysms larger than 7 mm in patients over 60 years of age.
The second crucial question is how to treat UIAs. Here, we will consider aneurysms of the anterior circulation, as endovascular treatment is almost always indicated in aneurysms of the posterior circulation. The aim of treatment should be to completely exclude the aneurysm with minimal morbidity. In hemorrhagic aneurysms, surgical treatment has higher morbidity [12] in the immediate postoperative period as well at annual follow-up than the endovascular method, however complete exclusion of the aneurysm is more permanent [3]. Considering UIA treatment modalities there is no quality prospective trial available, but from smaller studies [13] and their meta-analyses [14], more complete aneurysm exclusion in clipped groups with similar morbidity at annual follow-up has been shown. In particular, there is a notable difference in the quality of treatment in aneurysms of the MCA (complete exclusion of UIA 97% clip vs. 53% coil) [15].
The European Stroke Organization Guidelines [11] do not favor either method and emphasize an individual approach and consideration of each individual patient's risk factors (age, smoking status, previous subarachnoid hemorrhage, localization and aneurysm size, risk of treatment method for a specific type of aneurysm).
Comparison of individual publications regarding surgical treatment of UIAs is difficult primarily for two reasons. The first is various definitions of surgical morbidity. Worsening of neurological status (mRS > 2) is often considered an adverse neurological outcome. This very course definition of morbidity does not capture many patients that experience sustained neurological deficit due to surgery (while maintaining mRS < 2), in whom it is at least questionable whether the operation was beneficial (especially in aneurysms at low risk of rupture). Conversely, there are various analyses that may consider morbidity to be extended stay in a healthcare facility without identifying its cause, and the actual surgical morbidity may not be correctly represented. In better publications not only deterioration on the mRS or Glasgow Outcome Scale (GOS) are listed, but also any neurological, internal or surgical complication of the procedure that leads to prolonged hospital stay or the necessity for operative revision. Some publications report cognitive changes using the MMSE test or neuropsychological examinations in more detail. From the patient's perspective it is essential whether their clinical status is the same or better one year after the procedure, whether they can return to their previous way of life and whether the aneurysm has been completely excluded from the circulation. The mRS and GOS scales are very coarse in this respect, and any new or short-lasting deficits need to be included in permanent morbidity.
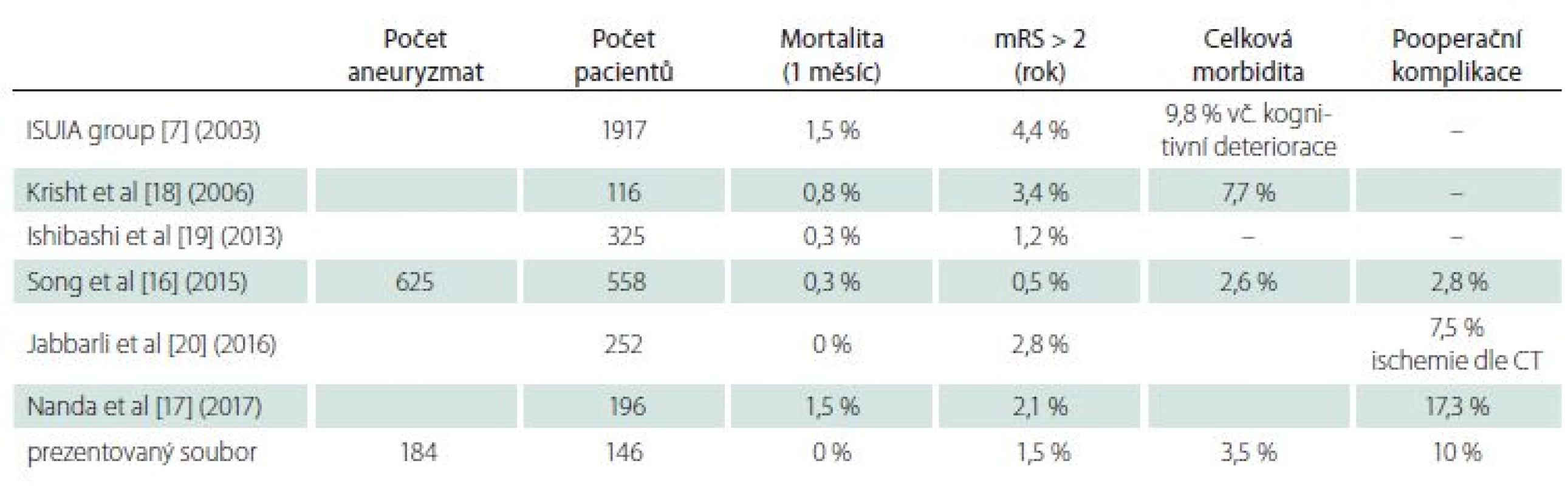
The second reason for the problematic comparison of publications is the differing composition of patients with different UIA operational difficulty. In a surgical subgroup (1,917 patients) from the multi-center prospective USUIA study published in 2003, the monthly mortality was 1.5% and annual morbidity (mRS > 2) was 4.4% (9.8% when cognitive deficits included) [6]. Better results have been achieved in the analysis of surgically-treated UIA patients at individual workplaces (Tab. 1) [16–21]. Excellent results from one center have been published on a group of 1,124 patients who were treated with a clip or coil (1 : 1) according to the decision of one physician (performing both types of procedure). Postoperative complications that did not lead to worsening of mRS (transient paresis of oculomotor nerve, subdural hematoma, intracerebral hematoma, epileptic seizure, etc.) were also included in total morbidity. The surgical group with 558 patients had a total morbidity of only 2.6%. Annual morbidity with mRS > 2 was 0.5% and monthly mortality 0.3% [16]. The endovascular group did not differ significantly. It should be emphasized that more than 50% of the aneurysms were smaller than 7 mm. A recently published article by Nanda et al. reported 196 patients with a mortality of 1.5% and morbidity 2.1%, with postoperative complications in 17.3% patients. Most of the aneurysms were larger than 1 cm [17]. Krisht et al. achieved a mortality rate of 0.8% and a permanent morbidity of 3.4%, with a transient morbidity of 7.7%, in 116 patients with aneurysms mostly larger than 7 mm [18].
In our sample of surgically-treated patients, 145 patients were examined after one year, 140 of whom underwent CTA. The postoperative early morbidity was 10%, the monthly mortality was zero and annual follow-up in 2 patients (1.5%) had unfavorable results (mRS > 2), while adjusting for any new deficit there were 5 patients (3.5%). The first patient with mRS > 2 was a 62-year-old woman with a complex 15 mm aneurysm of the ACoA, which we indicated primarily for coiling. A surgical approach was chosen due to high risk of the endovascular procedure. The aneurysm ruptured in the perioperative period, A2 was narrowed by the clip (ICG +) followed by the development of ischemia in the ACA territory resulting in clinically mild paresis of the lower extremity and mental disturbance (mRS 3). The second patient was a 37-year-old woman with a coincidental small aneurysm at the MCA bifurcation, had perioperative rupture of an unknown blister aneurysm of the ICA followed by ICA occlusion and resulting neurological deficit (mRS 3). Three other patients experienced new neurological deficit related to the surgical procedure after 1 year (2 ACoA with resulting mental slowing and one mild paresis after bypass with trapping in a complex aneurysm of the MCA). Of these 5 patients, 3 had ACoA aneurysms that were orientated superiorly or inferiorly with injury or occlusion of the perforating artery. In two of these patients, we considered endovascular treatment primarily in accordance with the conclusion of a meta-analysis of ACoA UIA treatment [21], however this approach was rejected due to high risk of complication and a surgical approach was chosen.
In our sample, complete exclusion of the aneurysm was achieved in 99% of patients. This number is higher than the commonly reported 90–95% [16] and is likely to be overestimated by the possible inaccuracy of CTA examinations that, despite the use of high-quality equipment and software, do not achieve the same accuracy as angiography. However, CTA has unquestionable advantages, mainly that it is non-invasive and thus the easy repeatable, as well as preoperative assessment of the relationship of the aneurysm to the cranial base which angiography does not offer. Post-operative examinations with titanium clips are affected to some extent by artifacts, which can however be greatly reduced by the use of metallic artifact reduction software to achieve high quality exams [22–24]. Minor residual flow may however be missed on CTA despite the reduction of artifacts, especially when using multiple clips, and it may be assumed that this may have happened in our sample. However, we did not find any changes after 4 years in comparison to the annual examination, which means that potential residual flow did not increase nor did any new aneurysms appear.
Conclusion
Microsurgical treatment of UIAs with the use of modern neurosurgical techniques allows the achievement of permanent exclusion of the aneurysm from the circulation with minimal morbidity in most patients. The development of microsurgical techniques and technology currently allows the treatment of most aneurysms from a small mini-pterional approach with careful intracranial dissection. Thus, treatment is becoming less invasive for the patient and is well tolerated.
Sources
1. Greving JP, Wermer MJ, Brown RD Jr et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 2014; 13(1): 59–66. doi: 10.1016/ S1474-4422(13)70263-1.
2. Etminan N, Brown RD Jr, Beseoglu K et al. The unruptured intracranial aneurysm treatment score: a multidisciplinary consensus. Neurology 2015; 85(10): 881–889. doi: 10.1212/ WNL.0000000000001891.
3. Spetzler RF, Zabramski JM, McDougall CG et al. Analysis of saccular aneurysms in the Barrow Ruptured Aneurysm Trial. J Neurosurg 2018; 128(1): 120–125. doi: 10.3171/ 2016.9.JNS161301.
4. Sun H, Safavi-Abbasi S, Spetzler RF. Retractorless surgery for intracranial aneurysms. J Neurosurg Sci 2016; 60(1): 54–69.
5. Konczalla J, Platz J, Fichtlscherer S et al. Rapid ventricular pacing for clip reconstruction of complex unruptured intracranial aneurysms: results of an interdisciplinary prospective trial. J Neurosurg 2018; 128(6): 1741–1752. doi: 10.3171/ 2016.11.JNS161420.
6. Vlak MH, Algra A, Brandenburg R et al. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 2011; 10(7): 626–636. doi: 10.1016/ S1474-4422(11)70109-0.
7. Wiebers DO, Whisnant JP, Huston J et al. International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured Intracranial Aneurysms: Natural History, Clinical Outcome, and Risks of Surgical and Endovascular Treatment. Lancet 2003; 362(9378): 103–110.
8. UCAS Japan Investigators, Morita A, Kirino T et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 2012; 366(26): 2474–2482. doi: 10.1056/ NEJMoa1113260.
9. Juvela S, Poussa K, Lehto H et al. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. Stroke 2013; 44(9): 2414–2421. doi: 10.1161/ STROKEAHA.113.001838.
10. Beneš V, Suchomel P. Mozková aneurysmata a subarachnoidální krvácení. Praha: Mladá Fronta 2017 : 166–167.
11. Steiner T, Juvela S, Unterberg A et al. European Stroke Organisation Guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis 2013; 35(2): 93–112. doi: 10.1159/ 000346087.
12. Molyneux AJ, Kerr RS, Yu LM et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366(9488): 809–817.
13. Darsaut TE, Findlay JM, Magro E et al. Surgical clipping or endovascular coiling for unruptured intracranial aneurysms: a pragmatic randomised trial. J Neurol Neurosurg Psychiatry 2017; 88(8): 663–668. doi: 10.1136/ jnnp-2016-315433.
14. Ruan C, Long H, Sun H et al. Endovascular coiling vs. surgical clipping for unruptured intracranial aneurysm: a meta-analysis. Br J Neurosurg 2015; 29(4): 485–492. doi: 10.3109/ 02688697.2015.1023771.
15. Smith TR, Cote DJ, Dasenbrock HH et al. Comparison of the efficacy and safety of endovascular coiling versus microsurgical clipping for unruptured middle cerebral artery aneurysms: a systematic review and meta-analysis. World Neurosurg 2015; 84(4): 942–953. doi: 10.1016/ j.wneu.2015.05.073.
16. Song J, Kim BS, Shin YS. Treatment outcomes of unruptured intracranial aneurysm; experience of 1,231 consecutive aneurysms. Acta Neurochir (Wien) 2015; 157(8): 1303–1311. doi: 10.1007/ s00701-015-2460-2.
17. Nanda A, Patra DP, Bir SC et al. Microsurgical clipping of unruptured intracranial aneurysms: a single surgeon‘s experience over 16 years. World Neurosurg 2017; 100 : 85–99. doi: 10.1016/ j.wneu.2016.12.099.
18. Krisht AF, Gomez J, Partington S. et al. Outcome of surgical clipping of unruptured aneurysms as it compares with a 10-year nonclipping survival period. Neurosurgery 2006; 58(2): 207–216.
19. Ishibashi T, Murayama Y, Saguchi T et al. Justification of unruptured intracranial aneurysm repair: a single--center experience. AJNR Am J Neuroradiol 2013; 34(8): 1600–1605. doi: 10.3174/ ajnr.A3470.
20. Jabbarli R, Wrede KH, Pierscianek D et al. Outcome after clipping of unruptured intracranial aneurysms depends on caseload. World Neurosurg 2016; 89 : 666–671. doi: 10.1016/ j.wneu.2015.12.043
21. O‘Neill AH, Chandra RV, Lai LT. Safety and effectiveness of microsurgical clipping, endovascular coiling, and stent assisted coiling for unruptured anterior communicating artery aneurysms: a systematic analysis of observational studies. J Neurointerv Surg 2017; 9(8): 761–765. doi: 10.1136/ neurintsurg-2016-012629.
22. Dunet V, Bernasconi M, Hajdu SD et al. Impact of metal artifact reduction software on image quality of gemstone spectral imaging dual-energy cerebral CT angiography after intracranial aneurysm clipping. Neuroradiology 2017; 59(9): 845–852. doi: 10.1007/ s00234-017-1871-6.
23. Kimura Y, Mikami T, Miyata K et al. Vascular assessment after clipping surgery using four-dimensional CT angiography. Neurosurg Rev 2019; 42(1): 107–114. doi: 10.1007/ s10143-018-0962-0.
24. Mamourian AC, Pluta DJ, Eskey CJ et al. Optimizing computed tomography to reduce artifacts from titanium aneurysm clips: an in vitro study. Technical note. J Neurosurg 2007; 107(6): 1238–1243.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2019 Issue 2
-
All articles in this issue
- Intradural extramedullary spinal cord tumors
- Multiple sclerosis and the role of gut microbiota during a harmful inflammatory response
- Genetic and neurobiological aspects of comorbid occurence of autism spectrum disorder and epilepsy
- Extra-intracranial bypass indicated during neurorehabilitation due to cognitive deterioration
- Traumatic pseudoaneurysm of the superficial temporal artery
- Multiple sclerosis, pregnancy, maternity, and breastfeeding
- Multiple sclerosis and pregnancy from a gynecologist‘s perspective – as sisted reproduction options
- Modern microsurgery as a permanent, safe and gentle solution of unruptured intracranial aneurysms
- Surgical explantation of a vagal nerve stimulator according to the magnetic resonance imaging protocol
- General movements and neurological development of the early age in children with neonatal hypoglycemia
- Comparison of cosmetic effects after short longitudinal and transverse skin incision for carotid endarterectomy
- Rapid diagnostics of chemokine CXCL13 in the cerebrospinal fluid of patients with neuroborreliosis
- Genetics of neuromuscular diseases
- Analýza dat v neurologii LXXIV. - Neparametrický Spearmanův koeficient korelace
- Recenze knih
- Doc. Vladimír Škorpil, 100 let od narození zakladatele naší elektromyografie
- Does leptin have a role in the development of intracranial meningiomas?
- A comparative study of myasthenic patients in the Czech and Slovak Republics
- Changes in essential and trace elements content in degenerating human intervertebral discs do not correspond to patients’ clinical status
- How extracellular sodium replacement affects the conduction velocity distribution of rats’ peripheral nerves
- Aneurysmal subarachnoid haemorrhage in pregnancy – successful clipping after coiling failure
- Tick-borne meningitis complicated by a cardioembolic intraluminal carotid artery thrombus and stroke
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Intradural extramedullary spinal cord tumors
- Rapid diagnostics of chemokine CXCL13 in the cerebrospinal fluid of patients with neuroborreliosis
- Genetics of neuromuscular diseases
- Multiple sclerosis and pregnancy from a gynecologist‘s perspective – as sisted reproduction options