Inpatient multidisciplinary rehabilitation programme for postural and gait stability in Huntington’s disease – a pilot study
Vliv multidisciplinárního rehabilitačního programu během hospitalizace na posturální stabilitu a stabilitu chůze u Huntingtonovy nemoci – pilotní studie
Cíl: Posturální instabilita a instabilita chůze patří mezi významné motorické symptomy Huntingtonovy nemoci (HN), které zvyšují riziko pádů. Rehabilitace je podstatnou součástí terapie instability. Cílem studie bylo zhodnotit, krátkodobý a dlouhodobý efekt multidisciplinárního rehabilitačního programu na posturální instabilitu i instabilitu chůze u HN a posoudit možnost provedení programu za hospitalizace.
Metodika: 13 pacientů s HN bez těžšího kognitivního deficitu a deprese absolvovalo třítýdenní multidisciplinární rehabilitační program během hospitalizace, který byl specificky zaměřený na posturální stabilitu a stabilitu chůze. Vyšetření proběhla na začátku programu, po dokončení rehabilitace, po 1 a 3 měsících od dokončení. Testování zahrnovalo vyšetření stability chůze (Dynamic Gait Index; DGI), posturální stability pomocí posturografu na stabilní (PSS) and nestabilní 20% (PSU) plošině a motorické skóre pomocí Unified Huntington’s Disease Rating Scale (UHDRS).
Výsledky: PSS prokázalo statisticky významné zlepšení přetrvávající po dobu 3 měsíců a signifikantní zlepšení v DGI ihned po rehabilitaci. V motorickém skóre UHDRS a PSU statisticky významné zlepšení nalezeno nebylo.
Závěr: Specifický rehabilitační program je bezpečný a dobře využitelný při terapii poruch stability u HN. Posturální stabilita dle PSS byla zlepšena po sledovanou dobu 3 měsíců. Zlepšení stability chůze dle DGI odeznělo do 1 měsíce. V PSU signifikantní zlepšení nebylo prokázáno. Tato studie nabízí návrh specifického rehabilitačního protokolu pro trénink stability u HN.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Klíčová slova:
Huntington’s disease – rehabilitation – gait – instability
Authors:
L. Brabcová 1,2; J. Roth 1; O. Ulmanová 1; J. Rusz 1,3; J. Klempíř 1; O. Horáček 2; M. Kolářová 2; P. Košková 2; P. Rolková 2; H. Božková 2; L. Sabó 2; M. Inemanová 2; K. Lísalová 2; F. Jančok 2; E. Růžička 1; H. Brožová 1
Authors place of work:
Department of Neurology and Centre of Clinical Neuroscience, Charles University, 1st Faculty of Medicine and General Teaching Hospital, Czech Republic
1; Rehabilitation Centre, Beroun Rehabilitation Hospital, Czech Republic
2; Department of Circuit Theory, Faculty of Electrical Engineering, Czech Technical University in Prague, Czech Republic
3
Published in the journal:
Cesk Slov Neurol N 2019; 82(3): 301-308
Category:
Původní práce
doi:
https://doi.org/10.14735/amcsnn2019301
Summary
Aim: Postural and gait instability in Huntington‘s disease (HD) is a key component of the motor symptomatology which contributes to an increased risk of falls. Rehabilitation is considered beneficial in postural and gait stability treatment. We aimed to explore the feasibility and the short - and long-term effects of an inpatient multidisciplinary rehabilitation program on postural and gait stability in subjects with HD.
Methods: A sample of 13 subjects with HD but with no severe cognitive deficit or depression underwent a 3-week specific inpatient rehabilitation program focused on postural and gait stability. Patients were examined at the baseline, after the completion of rehabilitation, and then 1 month and 3 months after the end of the program. The testing included: gait stability examination (Dynamic Gait Index; DGI), posturography examination of postural stability on a stable (PSS) and 20% unstable (PSU) platform and the total motor score evaluation by Unified Huntington‘s Disease Rating Scale (UHDRS).
Results: There was a significant improvement lasting 3 months in PSS and a significant improvement in DGI immediately after the rehabilitation. There was no significant improvement in the PSU and UHDRS total motor score.
Conclusion: Specific rehabilitation methods are safe and feasible and may be beneficial in the treatment of postural and gait instability in patients with early and mid-stage HD. The postural instability improvement measured by PSS persisted for at least 3 months. The gait stability improvement in DGI did not persist after 1 month. We found no improvement in PSU. This exploratory study offers a sample of a specific rehabilitation protocol for stability training in HD.
住院病人多学科康复计划的姿势和步态稳定亨廷顿病-一个试点研究
目的:
亨廷顿氏舞蹈症(HD)的姿势和步态不稳定是运动症状学的一个重要组成部分,它增加了跌倒的风险。康复被认为是有益的姿势和步态稳定治疗。我们的目的是探讨住院病人多学科康复计划对HD患者姿势和步态稳定性的可行性和短期和长期影响。
方法:
13名患有HD但没有严重认知缺陷或抑郁症的受试者接受了为期3周的特殊住院病人康复计划,重点是姿势和步态的稳定性。患者在基线、康复完成后、项目结束后1个月和3个月接受检查。测试包括:步态稳定性测试(动态步态指数;DGI)、稳定(PSS)和20%不稳定(PSU)平台上的姿势稳定性的足位造影检查,以及使用统一的亨廷顿氏病评分量表(UHDRS)进行运动总评分评估。
结果:
术后3个月PSS及DGI均有明显改善。PSU和UHDRS总分无明显改善。
结论:
特定的康复方法是安全可行的,可能有利于治疗早期和中期HD患者的姿势和步态不稳定。PSS测得的体位不稳定性改善至少持续3个月。DGI的步态稳定性改善在1个月后没有持续。我们发现PSU没有改善。本探索性研究提供了HD稳定训练的具体康复方案的样本。
关键词:
亨廷顿病-康复-步态-不稳定性
Keywords:
Huntingtonova nemoc – rehabilitace – chůze – instabilita
Introduction
Huntington’s disease (HD) is a hereditary neurodegenerative disease which manifests itself through involuntary movements and voluntary motor function impairment, together with cognitive and behavioral impairment.
The average age of HD onset is in the 4th decade, and the disease duration is commonly between 15–20 years. In the later stages it leads to complete disability and dependence on caregivers and the community. There is only limited and temporary symptomatic therapy [1]. Postural and gait instability in subjects with HD is a key component of the motor symptomatology [2], contributing to an increased risk of falls. Falls have been reported from 60 to 80% of subjects with HD [2–5]. Falls, injury and loss of independent ambulation are often factors which precipitate admission to a nursing home [6].
Subjects with HD manifest significant postural control deficits when performing motor skills typical of daily living activities [7]. Posturography shows a consistent pattern of abnormality in HD [8]. Significant deficits were reported in anticipatory postural adjustments [8,9], in reactive postural responses [10–12] as well as in still standing [10]. Subjects with HD showed considerably more anterior-posterior sway than normal, especially when visual and proprioceptive cues were eliminated [11]. Posturography examination of the limits of stability (LOS; the amount of maximum excursion an individual is able to cover intentionally in any direction without losing their balance or taking a step) showed impairment even before the clinical onset of the disease. The centre of pressure (COP) displacements were analyzed during maximum leaning in four basic directions and under three sensory conditions (eyes open, eyes closed and eyes closed standing on foam). Subjects with manifest HD showed significantly greater COP ranges than the healthy control subjects in all sensory conditions. The greatest deterioration was found when standing on foam [13].
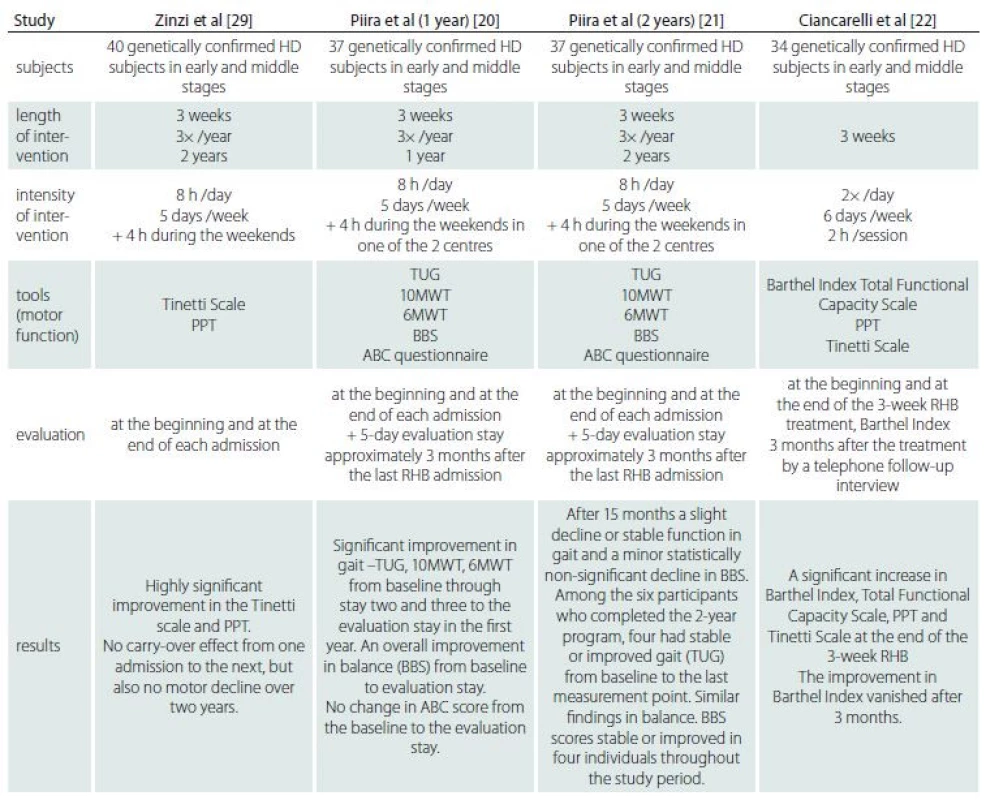
Rehabilitation may provide a therapeutic approach to stimulation of the brain to recruit alternative neuronal networks and enhance neuronal activity in pre-existing damaged neuronal networks [14]. Fritz et al in a recent systematic review found seven studies involving rehabilitation treatment and evaluation of balance in HD. It seems that rehabilitation may be beneficial in balance treatment [15]. Previous studies which focussed on both inpatient and outpatient rehabilitation programmes in HD showed the effects on motor performance, function (Activities of Daily Living; ADL) or general physical condition, and even improvements in the gait and balance of patients with HD [16–29]. However, there is a limited number of inpatient rehabilitation studies focussed specifically on postural and gait therapy in HD. Tab. 1 shows the methodology and results of comparable studies focussed on multidisciplinary inpatient rehabilitation using physiotherapy, occupational therapy, speech therapy, respiratory exercises, cognitive rehabilitation exercises, training in groups in the gym and/ or in a swimming pool, patient education sessions and group discussions for participants, assessment of the need for assistive devices and dietitian intervention.
Our study is the first prospective multidisciplinary study using a specific programme aimed at postural and gait stability in HD during a 3-week hospitalization. This study uses objective evaluation of postural and gait stability parameters repeatedly during a 4-month follow-up. A constant, clearly defined physiotherapy protocol focussed on postural and gait stability training with daily evaluation of improvement was used.
The aim of our study was to explore the feasibility and the short - and long-term effects of an inpatient multidisciplinary rehabilitation programme on postural and gait instability in the early and middle stages of HD.
Methods
Participants
The subjects with HD from The Movement Disorders Centre, Charles University in Prague were screened consecutively for the study during a 3-year period (2014–2016). The inclusion criteria were age over 18, genetically verified HD in the early and middle stages, stable medication, no other rehabilitation during the 4-month course of the study, and a signed informed consent form. The exclusion criteria were acute psychiatric symptoms, dementia preventing cooperation – Mini Mental State Examination (MMSE) [30] score lower than 20, concurrent depression – Beck Depression Inventory (BDI) [31] score higher than 9, severe immobility or co-morbidities preventing active cooperation in the rehabilitation programme or interfering with the monitored criteria (serious orthopaedic or internal medicine diagnoses, other neurological diagnoses causing movement impairment, severe visual impairment or hearing loss etc.), as well as non-compliance of the subject or the family. The study was performed in accordance with the ethical standards laid down in the 1975 Declaration of Helsinki. The responsible Institutional Review Boards approved the study protocol and the informed consent form (research Ethics Committee of the General University Hospital, Prague, approval number: 40/ 13).
Study design
The screening visit included a clinical neurological examination, which was performed by a neurologist experienced in HD, the MMSE test (a 30-point neuropsychological screening test for cognitive deficit; higher scores mean better performance) and the BDI test (a 21-question multiple-choice self-report inventory, a psychometric test for measuring the severity of depression; higher scores mean more severe depression) administered by
a neuropsychologist.
Outcome measures
The subjects were examined at the baseline, after the 3-week inpatient rehabilitation programme and 1 month and 3 months after finishing the programme.
The set of examinations included: a gait stability examination using the Dynamic Gait Index (DGI; assesses an individual’s ability to modify balance while walking in the presence of external demands – higher scores indicate better performance) [32], administered by a physician with a specialization in neurology and rehabilitation medicine and experienced in HD; the same examiner performed all the assessments and a postural stability examination by posturography, LOS [33], administered by a physiotherapist experienced in gait and posture; the same physiotherapist performed all the assessments. We used the Balance Master device and the Balance Master LOS Test. The LOS test quantifies the maximum distance the subject can intentionally displace their centre of gravity while maintaining stability. We measured the endpoint excursions in 8 directions, 4 cardinal + 4 diagonal, and their total scores. The subjects were asked to watch the screen and try to “hit the highlighted points with the cursor on the screen” by leaning their bodies. The test was performed once on the stable (PSS) and once on the 20% unstable (PSU) platform and the Unified Huntington’s Disease Rating Scale (UHDRS), the total motor score (a basic motor performance evaluation tool in HD – higher scores indicate lower performance) [34], administered by a neurologist experienced in HD who had completed the rater
training.
The whole examination process was conducted by a physician with a specialization in neurology and rehabilitation medicine and experienced in HD. Two subjects did not complete one of the follow-up posturography examinations.
Intervention
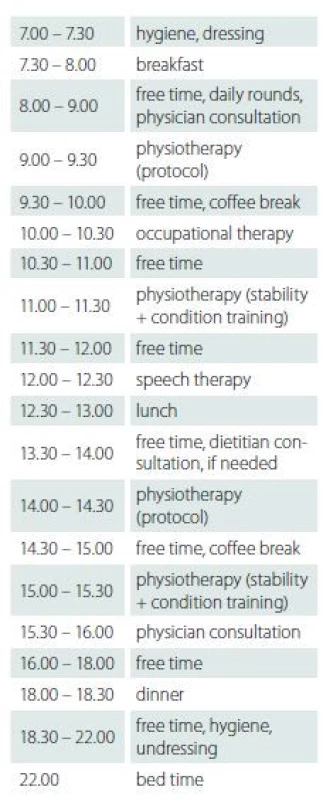
The 3-week inpatient rehabilitation programme included: 1. individual physiotherapy focussed on gait, stability and coordination according to a constant physiotherapy protocol (Tab. 2) twice a day for 30 min; 2. 60 min of other stability and condition training daily; 3. 30 min of occupational therapy daily, focussed mainly on motor coordination. No major tailoring was necessary as all subjects were able to take part in all of the activities. If the subject was tired, he/she was allowed to take a rest. If the task proved too difficult, it could be modified. In that case the task was assessed ’cannot do’ in the physiotherapy protocol.

The specific physiotherapy protocol was created in consideration of the typical stability and gait problems of subjects with HD (postural instability, impaired dynamic balance, impaired velocity control mechanisms, increased variability in temporal control [2,9,13,35–37]. The list of 20 exercises was evaluated every day (can/ cannot perform) to observe the changes in the separate tasks. ’Can perform’ was evaluated 1 point; ’cannot perform’ was evaluated at 0 points. As the list contains 20 exercises, the maximum was 20 points every day. The exercises were performed every day in a stable sequence by all subjects. The assessment was done during the therapy by the same physiotherapist during the whole stay. The task was assessed as ’can perform’ if the subject was able to do it without physical help. Verbal guidance was allowed.
In addition to the steady physiotherapy protocol (Tab. 2) 30 min twice a day, therapy with the physiotherapist included other 2× 30 min used for a workout on the ’Posturomed’ (Haider Bioswing, Pullenreuth, Germany) (a neuro-orthopaedic sensorimotor therapy and diagnosis device with an attenuated, oscillating, spring-dampened unstable platform; it is suspended on an oscillation frame that enables dosed, attenuated, compensating movements with variably adjusted oscillation amplitudes and frequencies), fitness workout (warming, stretching of hypertonic muscles, strength training of weakened muscles), aerobic exercise (stable bicycle, Nordic walking), exercise on the ’Motomed’ (Reck, Betzenweiler, Germany) (a neuro-orthopaedic upper and lower limb cycling device customized to be used passively, motor-assisted, or actively resistive; the subject sits on a stable chair and can follow visual feedback on a screen; the device was used as actively resistive during the training) and the ’DAVID system’ (DAVID Systems GmbH, Munich, Germany) (fitness machines for the treatment and monitoring of problems in the back, hips and knees, which are equipped with a monitoring unit that connects to a central server with a database of individual patients and their treatment outcomes and goals).
With the occupational therapists the subjects had 30 min a day of ADL training including the ’training flat’ (a place designed as a regular household for training in ADL and instrumental ADL with the aid of assistive devices and compensatory mechanisms), hand motor skill training, cognitive and executive function training (“The Happy Neuron – Brain Jogging” computer program [Alpelephant, s.r.o., Prague, Czech Republic] – an interactive cognitive stimulation tool designed for various medical and other backgrounds). The tasks were administered by the same occupational therapist during the entire course.
The subjects underwent speech and swallowing therapy and individual psychotherapy if needed. A social worker was available during the entire course together with an all-day nursing service. Tab. 3 shows an example of the daily programme.
Statistical analyses
For primary analysis, the Friedman test followed by Wilcoxon’s post hoc analyses were used to assess the subjects’ performance at the baseline, immediately post rehabilitation, and 1 month and 3 months after the rehabilitation programme. All follow-up examinations were compared to the
baseline.
Bonferroni correction for multiple comparisons was applied for 4 tests performed with a corrected P threshold equal to < 0.0125 (i.e. 0.05/ 4) for P < 0.05. For each type of exercise, the sum of successfully performed exercises (i.e. ’can perform’) was calculated separately for Day 1–9 and Day 10–18.
The effect of the individual exercises (i.e., secondary analysis) was calculated using the Wilcoxon signed rank sum test; no correction for multiple comparisons was applied.
Results
A sample of 16 genetically verified subjects with HD (9 women) in the early and middle stages were included in the rehabilitation programme. A group of 13 subjects completed the entire 4-month course of the study. Three subjects finished the 3-week inpatient programme but dropped out during the following 3 months of the follow-up. The mean age was 48 years (standard deviation [SD] 14, range 25–67), the mean HD duration was 6 years (SD 2.4, range 2–9) and the mean age of symptom onset was 42 years (SD 14, range 19–62). The mean number of triplets was 46 (SD 4.8, range 41–56). The mean Total Functional Capacity was 6.8
(SD 2.3, range 4–12, where 13 = normal and 0 = severe disability). One subject dropped out due to a concomitant medical condition (a leg fracture at home between visits) and two subjects due to personal organization and transportation problems. There were no adverse events during the therapy.
Fig. 1 and Tab. 4 show the subjects’ performance at baseline and immediately post rehabilitation, 1 month and 3 months after rehabilitation for measures of UHDRS, DGI, PSS and PSU (primary analysis).
*P < 0,05; **P < 0,01; x – odlehlé hodnoty
DGI – Dynamic Gait Index; PSS – posturální stabilita na stabilní plošině; PSU – posturální stabilita
na nestabilní plošině; UHDRS – Unifi ed Huntington’s Disease Rating Scale
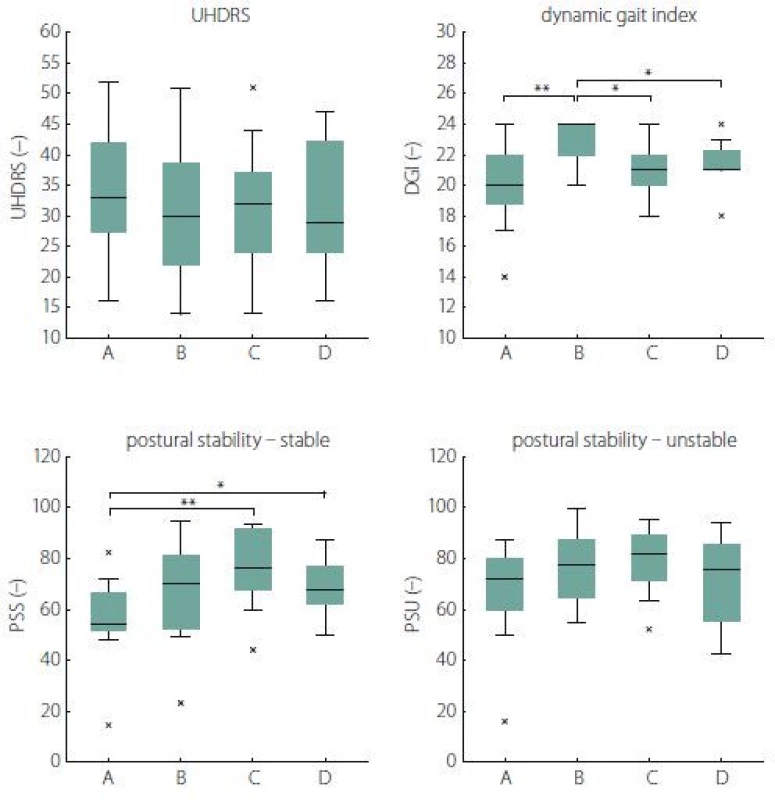
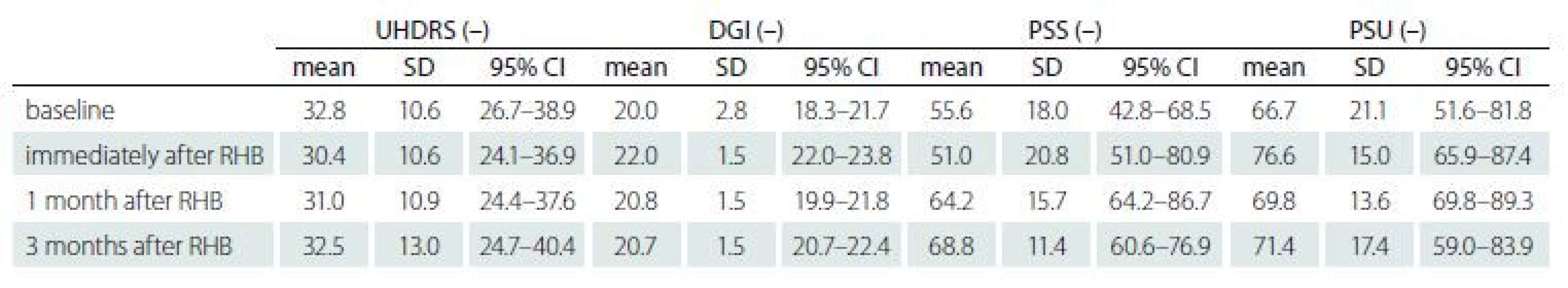
A significant change was found in PSS 2 (3.7) = 11.4, P = 0.04, W = 0.38, reflecting improvement 1 month after rehabilitation (P = 0.008, W = 1.00) as well as 3 months after rehabilitation (P = 0.04, W = 0.29) when compared to the baseline.
We also revealed a significant change in DGI λ2 (3.10) = 21.9, P < 0.001, W = 0.56, reflecting an improvement in the HD subjects’ performance from the baseline to the state immediately post rehabilitation (P = 0.008, W = 0.86). However, we did not find any significant changes in the next follow-up examinations when compared to the baseline.
No significant differences were found for the measure of UHDRS λ2 (3.10) = 5.1, P = 0.67, W = 0.13] as well as PSU λ2 (3.10) = 7.3, P = 0.25, W = 0.24].
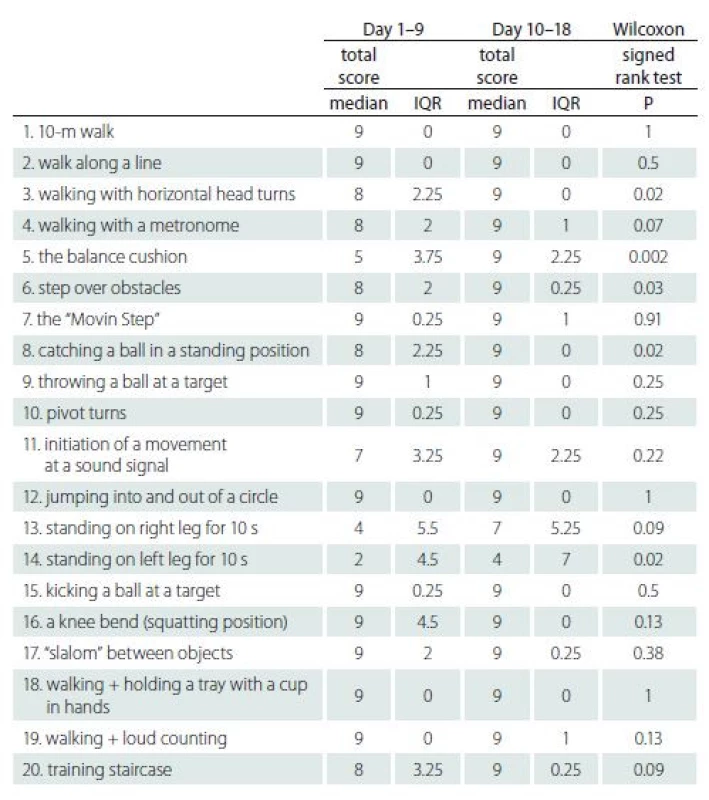
Tab. 5 lists the results of 20 individual exercises between Day 1–9 and Day 10–18. A significant improvement in the subjects’ performance was observed in the exercises of walking with horizontal head turns (P = 0.02), balance cushion (P = 0.002), stepping over obstacles (P = 0.03), catching a ball in a standing position (P = 0.02) and standing on the left leg for 10 s (P = 0.02).
Discussion
A limited number of studies included the question of how different types of rehabilitation methods and their frequency and intensity can influence the symptoms of HD.
Two systematic reviews evaluating physical therapy and exercise interventions in HD were published in 2017 [15,38]. They suggest that there is preliminary support for the benefits of exercise and physical activity in HD in terms of motor function, gait speed and balance, as well as a range of physical and social benefits identified through patient-reported outcomes. The reviews showed that the methods of subject recruitment, intensity of training and the physiotherapy methods used in individual studies were very diverse. This is why the comparison of the results with our study is quite complicated.
Our study is the first prospective multidisciplinary inpatient study using a specific programme aimed at postural and gait stability in HD. We found a significant improvement in DGI immediately after the rehabilitation programme. There was a decline both 1 month and 3 months after the rehabilitation. DGI is a very sensitive tool characterizing the subjects’ functional state. We did not find any comparable rehabilitation study using DGI in HD; however, there are studies using similar tools focussing on postural and gait stability.
Busse et al [16] demonstrated an improvement of the Romberg test outcomes in the intervention group compared to the control group. The scores on the Berg Balance Scale either improved after the rehabilitation intervention [20,23,25] or remained unchanged [18]. Subjects in the control group also demonstrated minor improvements [18]. Balance confidence on both walking and stairs measured by the Activities Balance Confidence Scale improved in the intervention group but declined in the control group [24]. There was no significant difference between the intervention group and the control group following a video-game balance intervention [28].
We found a significant improvement of PSS after the rehabilitation programme persisting for 1 as well as 3 months when compared to the baseline. There is no comparable rehabilitation study for the change in this parameter in HD. We found only a single study [37] using LOS on mechanically locked force plates. The study described a significant impairment of postural control (endpoint excursion, maximum excursion and directional control) in HD as compared to healthy controls.
We did not find any significant improvement in the PSU parameter. The study by Blanchet et al [13] proved a higher deterioration in PSU compared to PSS. We presume PSU is apparently a difficult test and the level of balance impairment in HD causes a certain limit in the ability to improve by means of rehabilitation.
The UHDRS total motor score did not show any improvement. The scale was used as the basic testing tool for subjects with HD. This scale contains only a few points aimed specifically at gait and stability, therefore we did not expect any significant improvement in this scale. Furthermore, we did not find any general progression of the disease during the 4-month period.
This study showed a significant performance improvement in several specific exercise items tested in the second part of the therapy in comparison with the first part. We can also see which exercise items were the most problematic for the majority of the subjects. It is interesting that they correlate mostly with those with a significant performance improvement. This could be considered in a future investigation of postural and gait stability in subjects with HD. The
20-point physiotherapy protocol might be utilized during the intervention.
The results of our study support the results reported by Zinzi et al [29], Piira et al [20,21] and Ciancarelli et al [22]. The studies show that intensive multidisciplinary rehabilitation may improve motor performance, including balance, in HD. The effect is obvious immediately after the intervention. We found carry-over effects in one stability parameter after 1 and 3 months. Piira et al [20,21] also described carry-over effects in gait and stability parameters between the admissions. We did not find any carry-over effects after 1 and 3 months in the other parameters, but there was also no decline during the whole course of the study, which is in accordance with the study performed by Zinzi et al [29].
One limitation of this study is the small sample size, because this is a single-centre study and HD is not a common disease. The performance and quality of cooperation might also be influenced by the extent of apathy or motivation in the HD
subjects.
Another limitation of the study is that if a multidisciplinary approach is used it might be difficult to delineate which exact method is responsible for the changes in the outcome measures. Some outcome measures might also be influenced by the training of the individual tasks during the intervention.
Randomized controlled clinical trials targeted at the effects of multidisciplinary intensive rehabilitation intervention on the progression of HD could bring us more information on what the optimal protocol of rehabilitation treatment in HD would be and which patients would profit most from the intervention. We are continuing with a longer follow-up and we are planning a comparison with a control group (subjects with HD without rehabilitation intervention).
Conclusion
Our findings suggest that specific rehabilitation methods may be beneficial in the treatment of postural and gait instability in early - and middle stage subjects with HD. An intensive inpatient multidisciplinary rehabilitation programme is safe, feasible and well-tolerated in motivated patients with HD.
The effect on postural stability tested by PSS persisted for at least 3 months. The improvement in gait stability tested by DGI did not remain after 1 month. There is a limit for the possibility of improvement in PSU, probably related to the degree of stability impairment in patients with HD. This study offers a sample of a specific rehabilitation protocol for stability training in patients with HD.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Accepted for review: 28. 2. 2019
Accepted for print: 18. 4. 2019
MUDr. Hana Brožová, Ph.D.
Department of Neurology and Centre of Clinical Neuroscience
1st Faculty of Medicine
Charles University and General University Hospital in
Prague
Kateřinská 30
128 21 Prague
Czech Republic
e-mail: hana.brozova@lf1.cuni.cz
Zdroje
1. Novak MJ, Tabrizi SJ. Huntington‘s disease. BMJ 2010; 340: c3109. doi: 10.1136/ bmj.c3109.
2. Busse ME, Wiles CM, Rosser AE. Mobility and falls in people with Huntington’s disease. J Neurol Neurosurg Psychiatry 2009; 80(1): 88–90. doi: 10.1136/ jnnp.2008.147
793.
3. Grimbergen YA, Knol MJ, Bloem BR et al. Falls and gait disturbances in Huntington‘s disease. Mov Disord 2008; 23(7): 970–976. doi: 10.1002/ mds.22003.
4. Williams S, Heron L, France K et al. Huntington‘s disease: characteristics of fallers. Physiother Res Int 2014; 10 : 1577. doi: 10.1002/ pri.1577.
5. Kloos AD, Kegelmeyer DA, Young GS et al. Fall risk assessment using the Tinetti mobility test in individuals with Huntington‘s disease. Mov Disord 2010; 25(16): 2838–2844. doi: 10.1002/ mds.23421.
6. Wheelock VL, Tempkin T, Marder K et al. Predictors of nursing home placement in Huntington disease. Neurology 2003; 60(6): 998–1001.
7. Panzera R, Salomonczyk D, Pirogovsky E et al. Postural deficits in Huntington‘s disease when performing motor skills involved in daily living. Gait Posture 2011; 33(3): 457–461. doi: 10.1016/ j.gaitpost.2010.12.025.
8. Delval A, Kryktowiak P, Blatt JL et al. A biomechanical study of gait initiation in Huntington’s disease. Gait Posture 2007; 25(2): 279–288. doi: 10.1016/ j.gaitpost.2006.04.001.
9. Delval A, Krystkowiak P, Blatt JL et al. Evolution of locomotor disorders in Huntington’s disease. Neurophysiol Clin 2008; 38(2): 117–125. doi: 10.1016/ j.neucli.2008.01.003.
10. Huttunen J, Hömberg V. EMG responses in leg muscles to postural pertubations in Huntington’s disease. J Neurol Neurosurg Psychiatry 1990; 53(1): 55–62.
11. Tian JR, Herdman SJ, Zee DS et al. Postural control in Huntington‘s disease (HD). Acta Otolaryngol 1991; 481 (Suppl): 333-336.
12. Goldberg A, Schepens SL, Feely ME et al. Deficits in stepping response time are associated with impairments in balance and mobility in people with Huntington disease. J Neurol Sci 2010; 298(1–2): 91–95. doi: 10.1016/ j.jns.2010.08.002.
13. Blanchet M, Prince F, Chouinard S et al. Postural stability limits in manifest and premanifest Huntington‘s disease under different sensory conditions. Neuroscience 2014; 279 : 102–112. doi: 10.1016/ j.neuroscience.2014.07.077.
14. Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol 2011; 7(2): 76–85. doi: 10.1038/ nrneurol.2010.200.
15. Fritz NE, Rao AK, Kegelmeyer D et al. Physical therapy and exercise interventions in Huntington‘s disease: a mixed methods systematic review. J Huntingtons Dis 2017; 6(3): 217–235. doi: 10.3233/ JHD-170260.
16. Busse M, Quinn L, Debono K et al. A randomized feasibility study of a 12-week community-based exercise program for people with Huntington’s disease. J Neurol Phys Ther 2013; 37(4): 149–158. doi: 10.1097/ NPT.00000 00000000016.
17. Busse M, Quinn L, Drew C et al. Physical activity self-management and coaching compared to social interaction in Huntington disease: results from the ENGAGE-HD randomized, controlled pilot feasibility trial. Phys Ther 2017; 97(6): 625–639. doi: 10.1093/ ptj/ pzx031.
18. Quinn L, Debono K, Dawes H et al. Task-specific training in Huntington disease: a randomized controlled feasibility trial. Phys Ther 2014; 94(11): 1555–1568. doi: 10.2522/ ptj.20140123.
19. Quinn L, Hamana K, Kelson M et al. A randomized, controlled trial of a multi-modal exercise intervention in Huntington‘s disease. Parkinsonism Relat Disord 2016; 31 : 46–52. doi: 10.1016/ j.parkreldis.2016.06.023.
20. Piira A, van Walsem MR, Mikalsen G et al. Effects of a one-year intensive multidisciplinary rehabilitation program for patients with Huntington‘s disease: a prospective intervention study. PLoS Curr 2013; 5. doi: 10.1371/ currents.hd.9504af71e0d1f87830c25c394be47
027.
21. Piira A, van Walsem MR, Mikalsen G et al. Effects of a two-year intensive multidisciplinary rehabilitation program for patients with Huntington‘s disease: a prospective intervention study. PLoS Curr 2014; 6. doi: 10.1371/ currents.hd.2c56ceef7f9f8e239a59ecf2d94cddac.
22. Ciancarelli I, Tozzi Ciancarelli MG, Carolei A. Effectiveness of intensive neurorehabilitation in patients with Huntington‘s disease. Eur J Phys Rehabil Med 2013; 49(2): 189–195.
23. Bohlen S, Ekwall C, Hellström K et al. Physical therapy in Huntington‘s disease – toward objective assessments? Eur J Neurol 2013; 20(2): 389–393. doi: 10.1111/ j.1468-1331.2012.03760.x.
24. Thompson JA, Cruickshank TM, Penailillo LE et al. The effects of multidisciplinary rehabilitation in patients with early-to-middle-stage Huntington‘s disease: a pilot study. Eur J Neurol 2013; 20(9): 1325–1329. doi: 10.1111/ ene.12053.
25. Mirek E, Filip M, Banaszkiewicz K et al. The effects of physiotherapy with PNF concept on gait and balance of patients with Huntington‘s disease – pilot study. Neurol Neurochir Pol 2015; 49(6): 354–357. doi: 10.1016/ j.pjnns.2015.09.002.
26. Clark D, Danzl MM, Ulanowski E. Development of a community-based exercise program for people diagnosed and at-risk for Huntington‘s disease: a clinical report. Physiother Theory Pract 2016; 32(3): 232–239. doi: 10.3109/ 09593985.2015.1110738.
27. Khalil H, Quinn L, van Deursen R et al. What effect does a structured home-based exercise programme have on people with Huntington‘s disease? A randomized, controlled pilot study. Clin Rehabil 2013; 27(7): 646–658. doi: 10.1177/ 0269215512473
762.
28. Kloos AD, Fritz NE, Kostyk SK et al. Video game play (Dance Dance Revolution) as a potential exercise therapy in Huntington‘s disease: a controlled clinical trial. Clin Rehabil 2013; 27(11): 972–982. doi: 10.1177/ 0269215513487235.
29. Zinzi P, Salmaso D, De Grandis R et al. Effects of an intensive rehabilitation programme on patients with Huntington‘s disease: a pilot study. Clin Rehabil 2007; 21(7): 603–613. doi: 10.1177/ 0269215507075495.
30. Folstein MF, Folstein SE, McHugh PR. „Mini-mental state“. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12(3): 189–198.
31. Beck AT, Ward CH, Mendelson M et al. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4(6): 561–571.
32. Raad J, Smiley J. MPH and the Rehabilitation measures team. Dynamic gait index. [online]. Available from URL: http: / / www.rehabmeasures.org.
33. Juras G, Słomka K, Fredyk A. Evaluation of the limits of stability (LOS) balance test. J Hum Kinet 2008; 19(1): 39–52. doi: 10.2478/ v10078-008-0003-0.
34. Huntington Study Group. Unified Huntington‘s Disease Rating Scale: reliability and consistency. Mov Disord 1996; 11(2): 136–142. doi: 10.1002/ mds.870110 204.
35. Rao AK, Muratori L, Louis ED. Spectrum of gait impairments in presymptomatic and symptomatic Huntington’s disease. Mov Disord 2008; 23(8): 1100–1107. doi: 10.1002/ mds.21987.
36. Rao AK, Muratori L, Louis ED et al. Clinical measurement of mobility and balance impairments in Huntington‘s disease: validity and responsiveness. Gait Posture 2009; 29(3): 433–436. doi: 10.1016/ j.gaitpost.2008.11.002.
37. Medina LD, Pirogovsky E, Salomonczyk D et al. Postural limits of stability in premanifest and manifest Huntington‘s disease. J Huntingtons Dis 2013; 2(2): 177–184. doi: 10.3233/ JHD-130048.
38. Quinn L, Busse M, Carrier J et al. Physical therapy and exercise interventions in Huntington’s disease: a mixed methods systematic review protocol. JBI Database System Rev Implement Rep 2017; 15(7): 1783–1799. doi: 10.11124/JBISRIR-2016-003274.
Štítky
Dětská neurologie Neurochirurgie NeurologieČlánek vyšel v časopise
Česká a slovenská neurologie a neurochirurgie

2019 Číslo 3
-
Všechny články tohoto čísla
- Editorial
- Neuromuscular diseases and pregnancy
- Are late complications of Parkinson’s disease really late? YES
- Are late complications of Parkinson’s disease really late? NO
- Are late complications of Parkinson’s disease really late? COMMENT
- Obstructive sleep apnea and cerebral blood flow
- Brief analysis of the frequency of use and spectrum of animal models in stroke research
- Factors affecting the school life of children with epilepsy
- Can endarterectomy of the external carotid artery be beneficial? A critical overview
- Circadian system disturbances in Huntington’s disease – implications for light therapy
- Experiences with an electrophysiological diagnosis of occupational ulnar nerve lesions at elbow
- Inpatient multidisciplinary rehabilitation programme for postural and gait stability in Huntington’s disease – a pilot study
- Optical coherence tomography measurements of the optic nerve head and retina in newly diagnosed idiopathic intracranial hypertension without loss of vision
- Coin in the Hand Test for detection of malingering memory impairment in comparison with mild cognitive impairment and mild dementia in Alzheimer‘s disease
- Neuropathic pain component in patients with myotonic dystrophy type 2 – a pilot study
- Equivalence of Montreal Cognitive Assessment alternate forms
- Frameless and fiducial-less method for deep brain stimulation
- Effect of vacuum-compression therapy for carpal tunnel syndrome as a part of physiotherapy – pilot study
- Anterior choroidal artery aneurysm
- Analýza dat v neurologii LXXV. Příklady chybné korelační analýzy
- Recenze knih
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle
- Coin in the Hand Test for detection of malingering memory impairment in comparison with mild cognitive impairment and mild dementia in Alzheimer‘s disease
- Neuromuscular diseases and pregnancy
- Optical coherence tomography measurements of the optic nerve head and retina in newly diagnosed idiopathic intracranial hypertension without loss of vision
- Effect of vacuum-compression therapy for carpal tunnel syndrome as a part of physiotherapy – pilot study
